BEAM or cyclophosphamide in autologous haematopoietic stem cell transplantation for relapsing-remitting multiple sclerosis
- PMID: 39187603
- PMCID: PMC11530369
- DOI: 10.1038/s41409-024-02397-x
BEAM or cyclophosphamide in autologous haematopoietic stem cell transplantation for relapsing-remitting multiple sclerosis
Abstract
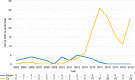
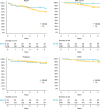
The most widely used conditioning regimens in autologous haematopoietic stem cell transplantation (ASCT) for multiple sclerosis (MS) are BEAM with anti-thymocyte globulin (ATG) and high-dose cyclophosphamide with ATG (Cy/ATG). In this retrospective study, we compare efficacy and safety of these regimens when used for relapsing-remitting MS. We assessed 231 patients treated in Sweden before January 1, 2020. The final cohort comprised 33 patients treated with BEAM/ATG and 141 with Cy/ATG. Prospectively collected data from the Swedish MS registry were used for efficacy, and electronic health records for procedure-related safety. The Kaplan-Meier estimate of 'no evidence of disease activity' (NEDA) at 5 years was 81% (CI 68-96%) with BEAM/ATG and 71% (CI 63-80%) with Cy/ATG, p = 0.29. Severe adverse events were more common with BEAM/ATG, mean 3.1 vs 1.4 per patient, p = <0.001. Febrile neutropaenia occurred in 88% of BEAM/ATG patients and 68% of Cy/ATG patients, p = 0.023. Average hospitalisation was 3.0 days longer in BEAM/ATG patients from day of stem-cell infusion, p < 0.001. While both regimens showed similar efficacy, BEAM/ATG was associated with more severe adverse events and prolonged hospitalisation. In the absence of randomised controlled trials, Cy/ATG may be preferable for ASCT in patients with relapsing-remitting MS due to its favourable safety profile.
© 2024. The Author(s).
Conflict of interest statement
EI has received speakers fee from Merck and honoraria from advisory boards for Sanofi-Aventis, Biogen and Merck. FP previously received research grants from Merck KGaA, Janssen and UCB outside this study. FP has received payment for expert testimony from Novartis. FP has participated in Data Monitoring Committee for clinical trials from Chugai, Lundbeck and Roche. JM has received lecture honorarium from Merck. NL has received honoraria from Sanofi. All other individual authors declare that there is no competing financial interests.
Figures

References
-
- McAllister LD, Beatty PG, Rose J. Allogeneic bone marrow transplant for chronic myelogenous leukemia in a patient with multiple sclerosis. Bone Marrow Transpl. 1997;19:395–7. - PubMed
-
- Yin JA, Jowitt SN. Resolution of immune-mediated diseases following allogeneic bone marrow transplantation for leukaemia. Bone Marrow Transpl. 1992;9:31–3. - PubMed
-
- Burt RK, Traynor AE, Pope R, Schroeder J, Cohen B, Karlin KH, et al. Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation. Blood. 1998;92:3505–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

