Single-cell analysis of chromatin and expression reveals age- and sex-associated alterations in the human heart
- PMID: 39187646
- PMCID: PMC11347658
- DOI: 10.1038/s42003-024-06582-y
Single-cell analysis of chromatin and expression reveals age- and sex-associated alterations in the human heart
Abstract
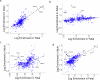
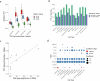
Sex differences and age-related changes in the human heart at the tissue, cell, and molecular level have been well-documented and many may be relevant for cardiovascular disease. However, how molecular programs within individual cell types vary across individuals by age and sex remains poorly characterized. To better understand this variation, we performed single-nucleus combinatorial indexing (sci) ATAC- and RNA-Seq in human heart samples from nine donors. We identify hundreds of differentially expressed genes by age and sex and find epigenetic signatures of variation in ATAC-Seq data in this discovery cohort. We then scale up our single-cell RNA-Seq analysis by combining our data with five recently published single nucleus RNA-Seq datasets of healthy adult hearts. We find variation such as metabolic alterations by sex and immune changes by age in differential expression tests, as well as alterations in abundance of cardiomyocytes by sex and neurons with age. In addition, we compare our adult-derived ATAC-Seq profiles to analogous fetal cell types to identify putative developmental-stage-specific regulatory factors. Finally, we train predictive models of cell-type-specific RNA expression levels utilizing ATAC-Seq profiles to link distal regulatory sequences to promoters, quantifying the predictive value of a simple TF-to-expression regulatory grammar and identifying cell-type-specific TFs. Our analysis represents the largest single-cell analysis of cardiac variation by age and sex to date and provides a resource for further study of healthy cardiac variation and transcriptional regulation at single-cell resolution.
© 2024. The Author(s).
Conflict of interest statement
C.T. is a SAB member, consultant and/or cofounder of Algen Biotechnologies, Altius Therapeutics, and Scale Biosciences. J.S. is a scientific advisory board member, consultant and/or cofounder of Cajal Neuroscience, Guardant Health, Maze Therapeutics, Camp4 Therapeutics, Phase Genomics, Adaptive Biotechnologies, and Scale Biosciences.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

