The PARP1 selective inhibitor saruparib (AZD5305) elicits potent and durable antitumor activity in patient-derived BRCA1/2-associated cancer models
- PMID: 39187844
- PMCID: PMC11348616
- DOI: 10.1186/s13073-024-01370-z
The PARP1 selective inhibitor saruparib (AZD5305) elicits potent and durable antitumor activity in patient-derived BRCA1/2-associated cancer models
Abstract
Background: Poly (ADP-ribose) polymerase 1 and 2 (PARP1/2) inhibitors (PARPi) are targeted therapies approved for homologous recombination repair (HRR)-deficient breast, ovarian, pancreatic, and prostate cancers. Since inhibition of PARP1 is sufficient to cause synthetic lethality in tumors with homologous recombination deficiency (HRD), PARP1 selective inhibitors such as saruparib (AZD5305) are being developed. It is expected that selective PARP1 inhibition leads to a safer profile that facilitates its combination with other DNA damage repair inhibitors. Here, we aimed to characterize the antitumor activity of AZD5305 in patient-derived preclinical models compared to the first-generation PARP1/2 inhibitor olaparib and to identify mechanisms of resistance.
Methods: Thirteen previously characterized patient-derived tumor xenograft (PDX) models from breast, ovarian, and pancreatic cancer patients harboring germline pathogenic alterations in BRCA1, BRCA2, or PALB2 were used to evaluate the efficacy of AZD5305 alone or in combination with carboplatin or an ataxia telangiectasia and Rad3 related (ATR) inhibitor (ceralasertib) and compared it to the first-generation PARPi olaparib. We performed DNA and RNA sequencing as well as protein-based assays to identify mechanisms of acquired resistance to either PARPi.
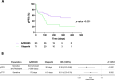
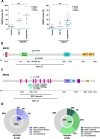
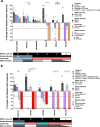
Results: AZD5305 showed superior antitumor activity than the first-generation PARPi in terms of preclinical complete response rate (75% vs. 37%). The median preclinical progression-free survival was significantly longer in the AZD5305-treated group compared to the olaparib-treated group (> 386 days vs. 90 days). Mechanistically, AZD5305 induced more replication stress and genomic instability than the PARP1/2 inhibitor olaparib in PARPi-sensitive tumors. All tumors at progression with either PARPi (39/39) showed increase of HRR functionality by RAD51 foci formation. The most prevalent resistance mechanisms identified were the acquisition of reversion mutations in BRCA1/BRCA2 and the accumulation of hypomorphic BRCA1. AZD5305 did not sensitize PDXs with acquired resistance to olaparib but elicited profound and durable responses when combined with carboplatin or ceralasertib in 3/6 and 5/5 models, respectively.
Conclusions: Collectively, these results show that the novel PARP1 selective inhibitor AZD5305 yields a potent antitumor response in PDX models with HRD and delays PARPi resistance alone or in combination with carboplatin or ceralasertib, which supports its use in the clinic as a new therapeutic option.
Keywords: Antitumor activity; BRCA1/2; Breast cancer; DNA damaging agent; HRD; Homologous recombination deficiency; PARP inhibitors; PARP1 selective; RAD51; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
ALG, VS, and JB are co-inventors of a patent related to this work (WO2019122411A1). We report research grants from AstraZeneca (VS) and personal fees and/or nonfinancial support from AstraZeneca (JB, VS), Pfizer (JB), and GSK (VS). ADS, FJCN, ST, LT, EL, AL, ALG, JF, MJOC, and MA are full time-time employees and shareholders at AZ. The remaining authors declare that they have no competing interests. The PARPi and ATRi used in this study were provided by AstraZeneca.
Figures


References
-
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. 10.1016/S1470-2045(14)70228-1 - DOI - PubMed
-
- Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–89. 10.1016/S1470-2045(16)30376-X - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2021 SGR 1510/Departament d'Empresa i Coneixement, Generalitat de Catalunya
- 2021 SGR 01112/Departament d'Empresa i Coneixement, Generalitat de Catalunya
- PI20/00892/Instituto de Salud Carlos III
- CPII19/00033/Instituto de Salud Carlos III
- PI19/01303/Instituto de Salud Carlos III
- PI22/01200/Instituto de Salud Carlos III
- INVES20095LLOP/Fundación Científica Asociación Española Contra el Cáncer
- POSTD211413AREN/Fundación Científica Asociación Española Contra el Cáncer
- SLT017/20/000081/PERIS - Departament de Salut, Generalitat de Catalunya
- LCF/BQ/DI19/11730051 (ID 100010434/La Caixa Foundation
- AGAUR 2020 FI-B 00584/Departament de Salut, Generalitat de Catalunya
- PERIS SLT028/23/000310/Departament de Salut, Generalitat de Catalunya
- PRE2021-099087/Ministerio de Ciencia, Innovación y Universidades
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

