Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis
- PMID: 39188132
- PMCID: PMC11462910
- DOI: 10.11124/JBIES-23-00291
Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis
Abstract
Objective: The objective of this review was to determine the diagnostic accuracy of the currently available and upcoming point-of-care rapid antigen tests (RATs) used in primary care settings relative to the viral genetic real-time reverse transcriptase polymerase chain reaction (RT-PCR) test as a reference for diagnosing COVID-19/SARS-CoV-2 in adults.
Introduction: Accurate COVID-19 point-of-care diagnostic tests are required for real-time identification of SARS-CoV-2 infection in individuals. Real-time RT-PCR is the accepted gold standard for diagnostic testing, requiring technical expertise and expensive equipment that are unavailable in most primary care locations. RATs are immunoassays that detect the presence of a specific viral protein, which implies a current infection with SARS-CoV-2. RATs are qualitative or semi-quantitative diagnostics that lack thresholds that provide a result within a short time frame, typically within the hour following sample collection. In this systematic review, we synthesized the current evidence regarding the accuracy of RATs for detecting SARS-CoV-2 compared with RT-PCR.
Inclusion criteria: Studies that included nonpregnant adults (18 years or older) with suspected SARS-CoV-2 infection, regardless of symptomology or disease severity, were included. The index test was any available SARS-CoV-2 point-of-care RAT. The reference test was any commercially distributed RT-PCR-based test that detects the RNA genome of SARS-CoV-2 and has been validated by an independent third party. Custom or in-house RT-PCR tests were also considered, with appropriate validation documentation. The diagnosis of interest was COVID-19 disease and SARS-CoV-2 infection. This review considered cross-sectional and cohort studies that examined the diagnostic accuracy of COVID-19/SARS-CoV-2 infection where the participants had both index and reference tests performed.
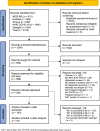
Methods: The keywords and index terms contained in relevant articles were used to develop a full search strategy for PubMed and adapted for Embase, Scopus, Qinsight, and the WHO COVID-19 databases. Studies published from November 2019 to July 12, 2022, were included, as SARS-CoV-2 emerged in late 2019 and is the cause of a continuing pandemic. Studies that met the inclusion criteria were critically appraised using QUADAS-2. Using a customized tool, data were extracted from included studies and were verified prior to analysis. The pooled sensitivity, specificity, positive predictive, and negative predictive values were calculated and presented with 95% CIs. When heterogeneity was observed, outlier analysis was conducted, and the results were generated by removing outliers.
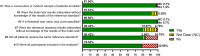
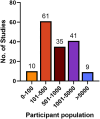
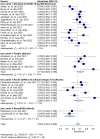
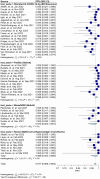
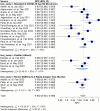
Results: Meta-analysis was performed on 91 studies of 581 full-text articles retrieved that provided true-positive, true-negative, false-positive, and false-negative values. RATs can identify individuals who have COVID-19 with high reliability (positive predictive value 97.7%; negative predictive value 95.2%) when considering overall performance. However, the lower level of sensitivity (67.1%) suggests that negative test results likely need to be retested through an additional method.
Conclusions: Most reported RAT brands had only a few studies comparing their performance with RT-PCR. Overall, a positive RAT result is an excellent predictor of a positive diagnosis of COVID-19. We recommend that Roche's SARS-CoV-2 Rapid Antigen Test and Abbott's BinaxNOW tests be used in primary care settings, with the understanding that negative results need to be confirmed through RT-PCR. We recommend adherence to the STARD guidelines when reporting on diagnostic data.
Review registration: PROSPERO CRD42020224250.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on Behalf of JBI.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD013705. doi: 10.1002/14651858.CD013705. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. PMID: 32845525 Free PMC article. Updated.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children.Nurs Rep. 2025 May 30;15(6):196. doi: 10.3390/nursrep15060196. Nurs Rep. 2025. PMID: 40559487 Free PMC article.
-
Comparative Analysis of RT-PCR and a Colloidal Gold Immunochromatographic Assay for SARS-CoV-2 Detection.Diagnostics (Basel). 2025 May 28;15(11):1362. doi: 10.3390/diagnostics15111362. Diagnostics (Basel). 2025. PMID: 40506934 Free PMC article.
References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard [internet]. World Health Organization; 2023. [cited 2023 Nov 8]. Available from: https://covid19.who.int/.
-
- Madhusoodanan J. Animal reservoirs—where the next SARS-CoV-2 variant could arise. JAMA 2022;328(8):696–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

