Molecular Mechanisms of Drosophila Hematopoiesis
- PMID: 39188265
- PMCID: PMC11345091
- DOI: 10.32607/actanaturae.27410
Molecular Mechanisms of Drosophila Hematopoiesis
Abstract
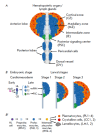
As a model organism, the fruit fly (Drosophila melanogaster) has assumed a leading position in modern biological research. The Drosophila genetic system has a number of advantages making it a key model in investigating the molecular mechanisms of metazoan developmental processes. Over the past two decades, significant progress has been made in understanding the molecular mechanisms regulating Drosophila hematopoiesis. This review discusses the major advances in investigating the molecular mechanisms involved in maintaining the population of multipotent progenitor cells and their differentiation into mature hemocytes in the hematopoietic organ of the Drosophila larva. The use of the Drosophila hematopoietic organ as a model system for hematopoiesis has allowed to characterize the complex interactions between signaling pathways and transcription factors in regulating the maintenance and differentiation of progenitor cells through the signals from the hematopoietic niche, autocrine and paracrine signals, and the signals emanated by differentiated cells.
Keywords: Drosophila melanogaster; differentiation; hematopoiesis; hematopoietic niche; hematopoietic organ; hematopoietic stem cells; hemocytes; multipotency; signaling pathways; transcription factors.
Copyright ® 2024 National Research University Higher School of Economics.
Figures

Similar articles
-
Drosophila as a Model to Study Cellular Communication Between the Hematopoietic Niche and Blood Progenitors Under Homeostatic Conditions and in Response to an Immune Stress.Front Immunol. 2021 Aug 16;12:719349. doi: 10.3389/fimmu.2021.719349. eCollection 2021. Front Immunol. 2021. PMID: 34484226 Free PMC article. Review.
-
Fatty acid β-oxidation is required for the differentiation of larval hematopoietic progenitors in Drosophila.Elife. 2020 Jun 12;9:e53247. doi: 10.7554/eLife.53247. Elife. 2020. PMID: 32530419 Free PMC article.
-
Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis.Development. 2010 Nov;137(21):3561-8. doi: 10.1242/dev.053728. Epub 2010 Sep 28. Development. 2010. PMID: 20876645 Free PMC article.
-
Intrinsic and Extrinsic Regulation of Hematopoiesis in Drosophila.Mol Cells. 2022 Mar 31;45(3):101-108. doi: 10.14348/molcells.2022.2039. Mol Cells. 2022. PMID: 35253654 Free PMC article. Review.
-
Hedgehog signaling from the Posterior Signaling Center maintains U-shaped expression and a prohemocyte population in Drosophila.Dev Biol. 2018 Sep 1;441(1):132-145. doi: 10.1016/j.ydbio.2018.06.020. Epub 2018 Jul 11. Dev Biol. 2018. PMID: 29966604 Free PMC article.
Cited by
-
Expansion of Drosophila haemocytes using a conditional GeneSwitch driver affects larval haemocyte function, but does not modulate adult lifespan or survival after severe infection.J Exp Biol. 2025 May 1;228(9):jeb249649. doi: 10.1242/jeb.249649. Epub 2025 May 6. J Exp Biol. 2025. PMID: 40116111 Free PMC article.
References
-
- Morgan T.H., Sci. Mon. 1935;41(1):5–18.
-
- Morgan T.H., Am. Nat. 1917;51(609):513–544.
-
- Nefedova L.N., Russ. J. Dev. Biol. 2020;51(4):201–211.
-
- Brand A.H., Perrimon N.. Development. 1993;118(2):401–415. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
