A Multidisciplinary Approach to Improve the Management of Immune-Checkpoint Inhibitor-Related Pneumonitis
- PMID: 39188308
- PMCID: PMC11346482
- DOI: 10.2147/OTT.S470892
A Multidisciplinary Approach to Improve the Management of Immune-Checkpoint Inhibitor-Related Pneumonitis
Abstract
Purpose: Treatment with immune-checkpoint inhibitors (ICIs) can be associated with a wide spectrum of immune-related adverse events (irAEs). Among irAEs, immune-mediated pneumonitis (im-PN) is a rare but potentially life-threatening side effect. TPrompt multidisciplinary diagnosis and effective management of im-PN may be essential to avoid severe complications and allowing resumation of therapy.
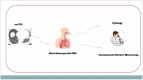
Patients and methods: We collected a case series of skin (melanoma, cutaneous squamous cell carcinoma-CSCC), lung, and mesothelioma cancer patients (pts), treated with ICI at the Center for Immuno-Oncology University Hospital of Siena, Italy, and diagnosed with im-PN. Clinical and radiologic data were thoroughly collected, as well as bronchoalveolar lavage (BAL) samples; im-PN was graded using CTCAE v. 5.0. Radiological patterns were reported according to the Fleischner Society classification.
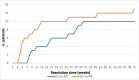
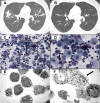
Results: From January 2014 to February 2023, 1004 patients with melanoma (522), CSCC (42), lung (342) or mesothelioma (98) were treated with ICI (619 monotherapy; 385 combination). Among treated patients, 24 (2%) developed an im-PN and 58% were symptomatic. Im-PN were classified as grades G1 (10) and G2 (14). Prompt steroid treatment led to complete resolution of im-PN in 21 patients, with a median time to resolution of 14 weeks (range: 0.4-51). Twelve patients resumed ICI therapy once fully-recovered and 2 experienced a recurrence that completely resolved with steroids after resumption of treatment. Three radiologic patterns were identified: organizational pneumonia-like (67%), pulmonary eosinophilia (29%), and hypersensitivity pneumonitis (4%). Furthermore, BAL analysis performed in 8 (33%) patients showed an inflammatory lymphocytic infiltrate, predominantly consisting of foam cell-like macrophage infiltrates in 6 cases. Notably, transmission electron microscopy evaluation performed in 2 patients revealed a scenario suggestive of a drug-mediated toxicity.
Conclusion: Im-PN is a rare but challenging side effect of ICI therapy, with variable time of onset and with heterogeneous clinical and radiological presentations. A multidisciplinary assessment is mandatory to optimize the clinical management of im-PN.
Keywords: cancer; immune-checkpoint inhibitors; immune-mediated pneumonitis; immunotherapy.
© 2024 Valente et al.
Conflict of interest statement
MV has served as a consultant e/o advisor to Novartis. MM has served as a consultant and/or advisor to Roche, Bristol Myers Squibb, Merck Sharp Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, GlaxoSmithKline, Sciclone, Sanofi, Alfasigma, Merck Serono, Novartis and iontura; and owns shares in Epigen Therapeutics, Srl. LC has served as consultant and/or advisor to Bristol Myers Squibb, AstraZeneca, Sanofi, Roche, and Merck Sharp Dohme, and has been compensated for educational activities by Bristol Myers Squibb, AstraZeneca and Sanofi. AMDG has served as a consultant and/or advisor to Incyte, Pierre Fabre, GlaxoSmithKline, Bristol Myers Squibb, Merck Sharp Dohme, and Sanofi and has been compensated for educational activities by Bristol Myers Squibb, Merck Sharp Dohme, Pierre Fabre and Sanofi. All other authors have no conflicts of interest to declare for this work.
Figures
References
LinkOut - more resources
Full Text Sources

