Augmenting osteoporotic bone regeneration through a hydrogel-based rejuvenating microenvironment
- PMID: 39188381
- PMCID: PMC11347042
- DOI: 10.1016/j.bioactmat.2024.07.036
Augmenting osteoporotic bone regeneration through a hydrogel-based rejuvenating microenvironment
Abstract
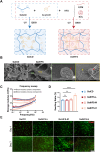
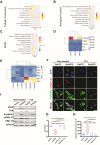
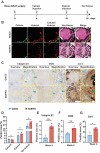
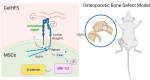
Osteoporotic bone defects pose a significant challenge for bone regeneration as they exhibit impaired healing capacity and delayed healing period. To address this issue, this study introduces a hydrogel that creates a rejuvenating microenvironment, thereby facilitating efficient bone repair during the initial two weeks following bone defect surgery. The hydrogel, named GelHFS, was created through host-guest polymerization of gelatin and acrylated β-cyclodextrin. Incorporation of the human fetal mesenchymal stem cell secretome (HFS) formed GelHFS hydrogel aimed at mimicking a rejuvenated stem cell niche. Our results demonstrated that GelHFS hydrogel promotes cell stellate spreading and osteogenic differentiation via integrin β1-induced focal adhesion pathway. Implantation of GelHFS hydrogel in an osteoporotic bone defect rat model recruited endogenous integrin β1-expressing cells and enhanced new bone formation and bone strength. Our findings reveal that GelHFS hydrogel provides a rejuvenating niche for endogenous MSCs and enhances bone regeneration in osteoporotic bone defect. These findings highlight the potential of GelHFS hydrogel as an effective therapeutic strategy for addressing challenging bone healing such as osteoporotic bone regeneration.
Keywords: Bone regeneration; Focal adhesion pathway; Osteoporotic bone defects; Rejuvenating microenvironment; Secretome.
© 2024 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article.
Figures




References
-
- Féron J.-M., Mauprivez R. Fracture repair: general aspects and influence of osteoporosis and anti-osteoporosis treatment. Injury. 2016;47:S10–S14. - PubMed
-
- Yaacobi E., Sanchez D., Maniar H., Horwitz D.S. Surgical treatment of osteoporotic fractures: an update on the principles of management. Injury. 2017;48(Suppl 7):S34–s40. - PubMed
-
- von Rüden C., Augat P. Failure of fracture fixation in osteoporotic bone. Injury. 2016;47(Suppl 2):S3–s10. - PubMed
-
- Cortet B. Bone repair in osteoporotic bone: postmenopausal and cortisone-induced osteoporosis. Osteoporos. Int. 2011;22(6):2007–2010. - PubMed
-
- Cheung W.H., Miclau T., Chow S.K.-H., Yang F.F., Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47:S21–S26. - PubMed
LinkOut - more resources
Full Text Sources

