Navigating treatment resistance: Janus kinase inhibitors for ulcerative colitis
- PMID: 39188602
- PMCID: PMC11269992
- DOI: 10.12998/wjcc.v12.i24.5468
Navigating treatment resistance: Janus kinase inhibitors for ulcerative colitis
Abstract
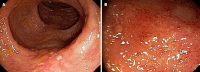
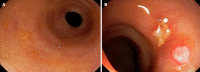
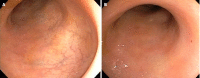
The management of refractory ulcerative colitis (UC) and acute severe UC (ASUC) is challenging due to the lack of standardized approaches in cases resistant to multiple treatments. In this editorial, I investigate the efficacy and safety of Janus kinase inhibitors, particularly upadacitinib and tofacitinib, in controlling severe and refractory disease. I highlight a notable case report by Xu et al, which explores the case of a patient with primary nonresponse to two classes of biologics and two fecal microbiota transplants who exhibited a remarkable response to upadacitinib. Furthermore, I discuss the use of tofacitinib in refractory UC and ASUC, either as monotherapy or in combination with biologics, which has shown promising response rates. Additionally, emerging evidence of upadacitinib efficacy in ASUC is presented. Overall, these cases emphasize the complex nature of managing refractory ASUC and the potential of small-molecule therapies to achieve remission. Further research is needed to refine treatment strategies for patients with treatment-resistant UC.
Keywords: Inflammatory bowel disease; Infliximab; Janus kinase inhibitor; Tofacitinib; Ulcerative colitis; Upadacitinib.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
References
-
- Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. - PubMed
-
- Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723–1736. - PubMed
-
- Peery AF, Kelly CR, Kao D, Vaughn BP, Lebwohl B, Singh S, Imdad A, Altayar O AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on Fecal Microbiota-Based Therapies for Select Gastrointestinal Diseases. Gastroenterology. 2024;166:409–434. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

