A cyanobacterial sigma factor F controls biofilm-promoting genes through intra- and intercellular pathways
- PMID: 39188729
- PMCID: PMC11345509
- DOI: 10.1016/j.bioflm.2024.100217
A cyanobacterial sigma factor F controls biofilm-promoting genes through intra- and intercellular pathways
Abstract
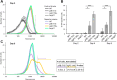
Cyanobacteria frequently constitute integral components of microbial communities known as phototrophic biofilms, which are widespread in various environments. Moreover, assemblages of these organisms, which serve as an expression platform, simplify harvesting the biomass, thereby holding significant industrial relevance. Previous studies of the model cyanobacterium Synechococcus elongatus PCC 7942 revealed that its planktonic growth habit results from a biofilm-suppression mechanism that depends on an extracellular inhibitor, an observation that opens the door to investigating cyanobacterial intercellular communication. Here, we demonstrate that the RNA polymerase sigma factor SigF1, is required for this biofilm-suppression mechanism whereas the S. elongatus paralog SigF2 is not involved in biofilm regulation. Comprehensive transcriptome analyses identified distinct regulons under the control of each of these sigma factors. sigF1 inactivation substantially lowers transcription of genes that code for the primary pilus subunit and consequently prevents pilus assembly. Moreover, additional data demonstrate absence of the biofilm inhibitor from conditioned medium of the sigF1 mutant, further validating involvement of the pilus assembly complex in secretion of the biofilm inhibitor. Consequently, expression is significantly upregulated for the ebfG-operon that encodes matrix components and the genes that encode the corresponding secretion system, which are repressed by the biofilm inhibitor in the wild type. Thus, this study uncovers a basic regulatory component of cyanobacterial intercellular communication, a field that is in its infancy. Elevated expression of biofilm-promoting genes in a sigF1 mutant supports an additional layer of regulation by SigF1 that operates via an intracellular mechanism.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Srivastava A., Summers M.L., Sobotka R. Cyanobacterial sigma factors: current and future applications for biotechnological advances. Biotechnol Adv. 2020;40 - PubMed
-
- Gruber T.M., Gross C.A. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. - PubMed
-
- Feklistov A., Sharon B.D., Darst S.A., Gross C.A. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol. 2014;68:357–376. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

