Impact of vaccination with third generation modified vaccinia Ankara and sexual behaviour on mpox incidence in men who have sex with men: analysis among participants of the ANRS-174 DOXYVAC trial
- PMID: 39188858
- PMCID: PMC11345388
- DOI: 10.1016/j.lanepe.2024.101020
Impact of vaccination with third generation modified vaccinia Ankara and sexual behaviour on mpox incidence in men who have sex with men: analysis among participants of the ANRS-174 DOXYVAC trial
Erratum in
-
Correction to "Impact of vaccination with third generation modified vaccinia Ankara and sexual behaviour on mpox incidence in men who have sex with men: analysis among participants of the ANRS-174 DOXYVAC trial" [The Lancet Regional Health-Europe 45, October 2024, 101020].Lancet Reg Health Eur. 2025 Apr 8;52:101297. doi: 10.1016/j.lanepe.2025.101297. eCollection 2025 May. Lancet Reg Health Eur. 2025. PMID: 40247951 Free PMC article.
Abstract
Background: Mpox was first reported in France on May 19 and third-generation live Modified Vaccinia Ankara (MVA-BN) vaccination of multiple-partner men who have sex with men (MSM) was recommended as of July 11, 2022. We assessed the impact of vaccination and of sexual behavior adopted during the epidemic period on mpox incidence in the ANRS-174-DOXYVAC trial enrolling MSM on HIV pre-exposure prophylaxis (PrEP) with history of sexually-transmitted infections (STI) in the previous year.
Methods: We compared pre-epidemic socio-behavioral characteristics and change in sexual behaviors after the onset of the epidemic of participants with mpox and mpox-free. Then we compared incidence rates of mpox per 1000 person-months (p-m) between May 9-July 10 (before vaccination of MSM, period-1) and July 11-September 20 2022 (after vaccination launch, period-2) and explored factors explaining the period effect using Poisson regression model.
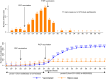
Findings: 472 MSM had data before and after May 9, 2022. Twenty percent had received smallpox vaccine during childhood. Mpox occurred in 77/472 participants (incidence 49.3 per 1000 p-m (95% CI 38.9-61.6)). MVA-BN vaccination roll-out was rapid, with 86% (341/398) of eligible participants having received at least one dose by September 20, 2022. Sexual behavior significantly changed before and after May 9, with a decrease in the proportion of mpox-free participants with >10 partners during last 3 months (45% vs 38%, p = 0.0035). Mpox incidence was 67.4 per 1000 p-m (95% CI 51.6-86.6) in period-1, and 24.4 per 1000 p-m (95% CI 13.9-39.6) in period-2, with an incidence rate ratio of 0.36 (95% CI 0.21-0.63). In multivariable Poisson regression model, only MVA-BN vaccination in 2022 remained significantly associated with mpox incidence, with a 99% risk reduction (95% CI 96.6-99.7).
Interpretation: In MSM on PrEP enrolled in the ANRS-174-DOXYVAC trial, rapid roll-out of MVA-BN vaccination was associated with a strong reduction in mpox incidence.
Funding: ANRS Maladies Infectieuses Emergentes (ANRS/MIE).
Keywords: Incidence; Mitigation; Mpox; Outbreak; Roll-out; Sexual behavior; Vaccination.
© 2024 The Author(s).
Conflict of interest statement
Dr Ghosn received consulting fees from Gilead and ViiV Healthcare. Dr Duvivier received consulting fees from Gilead and support for attending meetings and/or travel from Gilead, Merck and ViiV Healthcare. Dr Costagliola received honoraria for lecture from Pfizer. Dr Molina received grants from Gilead and Merck, consulting fees from Gilead, Merck and ViiV Healthcare and payment for participation on a DSMB from Aelix. All other authors have nothing to declare.
Figures
References
-
- Durski K.N., McCollum A.M., Nakazawa Y., et al. Emergence of monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. Erratum in: MMWR Morb Mortal Wkly Rep. 2018 Apr 27;67(16):479. PMID: 29543790; PMCID: PMC5857192. - DOI - PMC - PubMed
-
- Girometti N., Byrne R., Bracchi M., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22(9):1321–1328. doi: 10.1016/S1473-3099(22)00411-X. Epub 2022 Jul 1. PMID: 35785793; PMCID: PMC9534773. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous