Telemonitoring of Active Inflammatory Bowel Disease Using the App TECCU: Short-Term Results of a Multicenter Trial of GETECCU
- PMID: 39189160
- PMCID: PMC11612589
- DOI: 10.2196/60966
Telemonitoring of Active Inflammatory Bowel Disease Using the App TECCU: Short-Term Results of a Multicenter Trial of GETECCU
Abstract
Background: Telemonitoring for inflammatory bowel disease (IBD) has not consistently demonstrated superiority over standard care; however, noninferiority may be an acceptable outcome if remote care proves to be more efficient.
Objective: This study aims to compare the remission time and quality of life of patients with active IBD managed through standard care versus the TECCU (Telemonitoring of Crohn Disease and Ulcerative Colitis) app.
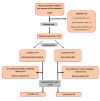
Methods: A 2-arm, randomized, multicenter trial with a noninferiority design was conducted across 24 hospitals in Spain. The study included adult patients with IBD who were starting immunosuppressive or biological therapy. Participants were randomized into 2 groups: the telemonitoring group (G_TECCU) and the standard care group (G_Control). The follow-up schedule for the telemonitoring group (G_TECCU) was based on contacts via the TECCU app, while the control group (G_Control) adhered to standard clinical practice, which included in-person visits and telephone calls. In both groups, treatment adjustments were made based on the progression of disease activity and medication adherence, assessed using specific indices and biological markers at each check-up. The primary outcome was the duration of remission after 12 weeks, while secondary outcomes included quality of life, medication adherence, adverse events, and patient satisfaction.
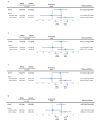
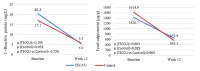
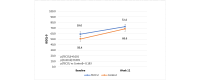
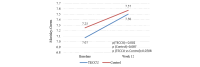
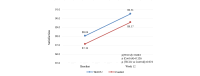
Results: Of the 169 patients enrolled, 158 were randomized and 150 were analyzed per protocol: telemonitoring (n=71) and control (n=79). After 12 weeks, the time in clinical remission was not inferior in the telemonitoring group (mean 4.20, SD 3.73 weeks) compared with the control group (mean 4.32, SD 3.28 weeks), with a mean difference between arms of -0.12 weeks (95% CI -1.25 to 1.01; noninferiority P=.02). The mean reduction in C-reactive protein values was -15.40 mg/L (SD 90.15 mg/L; P=.19) in the G_TECCU group and -13.16 mg/L (SD 54.61 mg/L; P=.05) in the G_Control group, with no significant differences between the 2 arms (P=.73). Similarly, the mean improvement in fecal calprotectin levels was 832.3 mg/L (SD 1825.0 mg/L; P=.003) in the G_TECCU group and 1073.5 mg/L (SD 3105.7 mg/L; P=.03) in the G_Control group; however, the differences were not statistically significant (P=.96). Quality of life improved in both groups, with a mean increase in the 9-item Inflammatory Bowel Disease Questionnaire score of 13.44 points (SD 19.1 points; P<.001) in the G_TECCU group and 18.23 points (SD 22.9 points; P=.001) in the G_Control group. Additionally, the proportion of patients who adhered to their medication significantly increased from 35% (25/71) to 68% (48/71) in the G_TECCU group (P=.001) and from 46% (36/79) to 73% (58/79) in the G_Control group (P=.001). The satisfaction rate remained stable at around 90%, although noninferiority was not demonstrated for the secondary outcomes.
Conclusions: Telemonitoring patients with active IBD is not inferior to standard care for achieving and maintaining short-term remission. The TECCU app may serve as a viable alternative follow-up tool, pending confirmation of improved health outcomes and cost-effectiveness over the long-term.
Trial registration: ClinicalTrials.gov NCT06031038; https://clinicaltrials.gov/ct2/show/NCT06031038.
International registered report identifier (irrid): RR2-10.2196/resprot.9639.
Keywords: Crohn disease; clinical trial; health outcomes; inflammatory bowel disease; quality of life; remission time; socioeconomical and psychological end points; telemonitoring; ulcerative colitis.
©Mariam Aguas, Javier Del Hoyo, Raquel Vicente, Manuel Barreiro-de Acosta, Luigi Melcarne, Alejandro Hernandez-Camba, Lucía Madero, María Teresa Arroyo, Beatriz Sicilia, María Chaparro, María Dolores Martin-Arranz, Ramón Pajares, Francisco Mesonero, Miriam Mañosa, Pilar Martinez, Silvia Chacón, Joan Tosca, Sandra Marín, Luciano Sanroman, Marta Calvo, David Monfort, Empar Saiz, Yamile Zabana, Ivan Guerra, Pilar Varela, Virginia Baydal, Raquel Faubel, Pilar Corsino, Sol Porto-Silva, Eduard Brunet, Melodi González, Ana Gutiérrez, Pilar Nos. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 18.11.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Kappelman MD, Porter CQ, Galanko JA, Rifas-Shiman SL, Ollendorf DA, Sandler RS, Finkelstein JA. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011 Jan;17(1):62–8. doi: 10.1002/ibd.21371. https://europepmc.org/abstract/MED/20564532 - DOI - PMC - PubMed
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017 Dec 23;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0.S0140-6736(17)32448-0 - DOI - PubMed
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001.S0016-5085(11)01378-3 - DOI - PubMed
-
- Chaparro M, Garre A, Núñez Ortiz Andrea, Diz-Lois Palomares María Teresa, Rodríguez Cristina, Riestra S, Vela Milagros, Benítez José Manuel, Fernández Salgado Estela, Sánchez Rodríguez Eugenia, Hernández Vicent, Ferreiro-Iglesias Rocío, Ponferrada Díaz Ángel, Barrio Jesús, Huguet José María, Sicilia Beatriz, Martín-Arranz María Dolores, Calvet Xavier, Ginard Daniel, Alonso-Abreu Inmaculada, Fernández-Salazar Luis, Varela Trastoy Pilar, Rivero Montserrat, Vera-Mendoza Isabel, Vega Pablo, Navarro Pablo, Sierra Mónica, Cabriada José Luis, Aguas Mariam, Vicente Raquel, Navarro-Llavat Mercè, Echarri Ana, Gomollón Fernando, Guerra Del Río Elena, Piñero Concepción, Casanova María José, Spicakova Katerina, Ortiz de Zarate Jone, Torrella Cortés Emilio, Gutiérrez Ana, Alonso-Galán Horacio, Hernández-Martínez Álvaro, Marrero José Miguel, Lorente Poyatos Rufo, Calafat Margalida, Martí Romero Lidia, Robledo Pilar, Bosch Orencio, Jiménez Nuria, Esteve Comas María, Duque José María, Fuentes Coronel Ana María, Josefa Sampedro Manuela, Sesé Abizanda Eva, Herreros Martínez Belén, Pozzati Liliana, Fernández Rosáenz Hipólito, Crespo Suarez Belén, López Serrano Pilar, Lucendo Alfredo J, Muñoz Vicente Margarita, Bermejo Fernando, Ramírez Palanca José Joaquín, Menacho Margarita, Carmona Amalia, Camargo Raquel, Torra Alsina Sandra, Maroto Nuria, Nerín de la Puerta Juan, Castro Elena, Marín-Jiménez Ignacio, Botella Belén, Sapiña Amparo, Cruz Noelia, Forcelledo José Luis F, Bouhmidi Abdel, Castaño-Milla Carlos, Opio Verónica, Nicolás Isabel, Kutz Marcos, Abraldes Bechiarelli Alfredo, Gordillo Jordi, Ber Yolanda, Torres Domínguez Yolanda, Novella Durán María Teresa, Rodríguez Mondéjar Silvia, Martínez-Cerezo Francisco J, Kolle Lilyan, Sabat Miriam, Ledezma Cesar, Iyo Eduardo, Roncero Óscar, Irisarri Rebeca, Lluis Laia, Blázquez Gómez Isabel, Zapata Eva María, José Alcalá María, Martínez Pascual Cristina, Montealegre María, Mata Laura, Monrobel Ana, Hernández Camba Alejandro, Hernández Luis, Tejada María, Mir Alberto, Galve María Luisa, Soler Marta, Hervías Daniel, Gómez-Valero José Antonio, Barreiro-de Acosta Manuel, Rodríguez-Artalejo Fernando, García-Esquinas Esther, Gisbert Javier P, The EpidemIBD Study Group Of Geteccu Incidence, clinical characteristics and management of inflammatory bowel disease in Spain: large-scale epidemiological study. J Clin Med. 2021 Jun 29;10(13):2885. doi: 10.3390/jcm10132885. https://www.mdpi.com/resolver?pii=jcm10132885 jcm10132885 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

