Canonical and Non-Canonical Wnt Signaling Generates Molecular and Cellular Asymmetries to Establish Embryonic Axes
- PMID: 39189260
- PMCID: PMC11348223
- DOI: 10.3390/jdb12030020
Canonical and Non-Canonical Wnt Signaling Generates Molecular and Cellular Asymmetries to Establish Embryonic Axes
Abstract
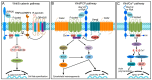
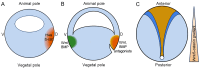
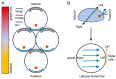
The formation of embryonic axes is a critical step during animal development, which contributes to establishing the basic body plan in each particular organism. Wnt signaling pathways play pivotal roles in this fundamental process. Canonical Wnt signaling that is dependent on β-catenin regulates the patterning of dorsoventral, anteroposterior, and left-right axes. Non-canonical Wnt signaling that is independent of β-catenin modulates cytoskeletal organization to coordinate cell polarity changes and asymmetric cell movements. It is now well documented that components of these Wnt pathways biochemically and functionally interact to mediate cell-cell communications and instruct cellular polarization in breaking the embryonic symmetry. The dysfunction of Wnt signaling disrupts embryonic axis specification and proper tissue morphogenesis, and mutations of Wnt pathway genes are associated with birth defects in humans. This review discusses the regulatory roles of Wnt pathway components in embryonic axis formation by focusing on vertebrate models. It highlights current progress in decoding conserved mechanisms underlying the establishment of asymmetry along the three primary body axes. By providing an in-depth analysis of canonical and non-canonical pathways in regulating cell fates and cellular behaviors, this work offers insights into the intricate processes that contribute to setting up the basic body plan in vertebrate embryos.
Keywords: Spemann organizer; Wnt signaling; anteroposterior axis; asymmetry formation; axis formation; dorsoventral axis; laterality defects; left–right axis; left–right organizer.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

