Neurophysiological markers of motor compensatory mechanisms in early Parkinson's disease
- PMID: 39189320
- PMCID: PMC11531851
- DOI: 10.1093/brain/awae210
Neurophysiological markers of motor compensatory mechanisms in early Parkinson's disease
Abstract
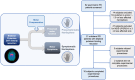
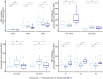
Compensatory mechanisms in Parkinson's disease are defined as the changes that the brain uses to adapt to neurodegeneration and progressive dopamine reduction. Motor compensation in early Parkinson's disease could, in part, be responsible for a unilateral onset of clinical motor signs despite the presence of bilateral nigrostriatal degeneration. Although several mechanisms have been proposed for compensatory adaptations in Parkinson's disease, the underlying pathophysiology is unclear. Here, we investigate motor compensation in Parkinson's disease by investigating the relationship between clinical signs, dopamine transporter imaging data and neurophysiological measures of the primary motor cortex (M1), using transcranial magnetic stimulation in presymptomatic and symptomatic hemispheres of patients. In this cross-sectional, multicentre study, we screened 82 individuals with Parkinson's disease. Patients were evaluated clinically in their medication OFF state using standardized scales. Sixteen Parkinson's disease patients with bilateral dopamine transporter deficit in the putamina but unilateral symptoms were included. Twenty-eight sex- and age-matched healthy controls were also investigated. In all participants, we tested cortical excitability using single- and paired-pulse techniques, interhemispheric inhibition and cortical plasticity with paired associative stimulation. Data were analysed with ANOVAs, multiple linear regression and logistic regression models. Individual coefficients of motor compensation were defined in patients based on clinical and imaging data, i.e. the motor compensation coefficient. The motor compensation coefficient includes an asymmetry score to balance motor and dopamine transporter data between the two hemispheres, in addition to a hemispheric ratio accounting for the relative mismatch between the magnitude of motor signs and dopaminergic deficit. In patients, corticospinal excitability and plasticity were higher in the presymptomatic compared with the symptomatic M1. Also, interhemispheric inhibition from the presymptomatic to the symptomatic M1 was reduced. Lower putamen binding was associated with higher plasticity and reduced interhemispheric inhibition in the presymptomatic hemisphere. The motor compensation coefficient distinguished the presymptomatic from the symptomatic hemisphere. Finally, in the presymptomatic hemisphere, a higher motor compensation coefficient was associated with lower corticospinal excitability and interhemispheric inhibition and with higher plasticity. In conclusion, the present study suggests that motor compensation involves M1-striatal networks and intercortical connections becoming more effective with progressive loss of dopaminergic terminals in the putamen. The balance between these motor networks seems to be driven by cortical plasticity.
Keywords: Parkinson’s disease; cortical plasticity; dopamine transporter compensatory mechanisms.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
All authors declare no competing interests. R.C. has received speaking honoraria from Zambon Italia; Zambon SAU; Bial Italia Srl; Advisory board fees from Bial; Research support from the Ministero Italiano della Salute; Editor-in-Chief of the neuromuscular and movement disorders section of
Figures



Comment in
-
Motor compensation in Parkinson's disease: an empirical challenge with clinical implications.Brain. 2024 Nov 4;147(11):3648-3650. doi: 10.1093/brain/awae328. Brain. 2024. PMID: 39414256 No abstract available.
References
-
- Foffani G, Obeso JA. A cortical pathogenic theory of Parkinson’s disease. Neuron. 2018;99:1116–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

