CRISPR-Cas target recognition for sensing viral and cancer biomarkers
- PMID: 39189452
- PMCID: PMC11417378
- DOI: 10.1093/nar/gkae736
CRISPR-Cas target recognition for sensing viral and cancer biomarkers
Abstract
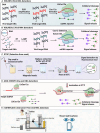
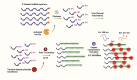
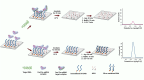
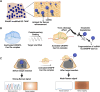
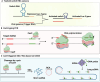
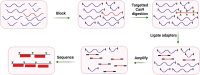
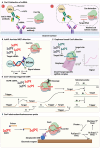
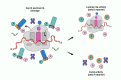
Nucleic acid-based diagnostics is a promising venue for detection of pathogens causing infectious diseases and mutations related to cancer. However, this type of diagnostics still faces certain challenges, and there is a need for more robust, simple and cost-effective methods. Clustered regularly interspaced short palindromic repeats (CRISPRs), the adaptive immune systems present in the prokaryotes, has recently been developed for specific detection of nucleic acids. In this review, structural and functional differences of CRISPR-Cas proteins Cas9, Cas12 and Cas13 are outlined. Thereafter, recent reports about applications of these Cas proteins for detection of viral genomes and cancer biomarkers are discussed. Further, we highlight the challenges associated with using these technologies to replace the current diagnostic approaches and outline the points that need to be considered for designing an ideal Cas-based detection system for nucleic acids.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas Advancement in Molecular Diagnostics and Signal Readout Approaches.J Mol Diagn. 2021 Nov;23(11):1433-1442. doi: 10.1016/j.jmoldx.2021.07.025. Epub 2021 Aug 25. J Mol Diagn. 2021. PMID: 34454111 Review.
-
Applications of the CRISPR-Cas system for infectious disease diagnostics.Expert Rev Mol Diagn. 2021 Jul;21(7):723-732. doi: 10.1080/14737159.2021.1922080. Epub 2021 May 18. Expert Rev Mol Diagn. 2021. PMID: 33899643 Review.
-
CRISPR/Cas systems for the detection of nucleic acid and non-nucleic acid targets.Nano Res. 2023 Mar 20:1-14. doi: 10.1007/s12274-023-5567-4. Online ahead of print. Nano Res. 2023. PMID: 37359078 Free PMC article. Review.
-
Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry.Genes (Basel). 2022 Nov 2;13(11):2007. doi: 10.3390/genes13112007. Genes (Basel). 2022. PMID: 36360244 Free PMC article. Review.
-
RNA-Targeting CRISPR-Cas Systems and Their Applications.Int J Mol Sci. 2020 Feb 7;21(3):1122. doi: 10.3390/ijms21031122. Int J Mol Sci. 2020. PMID: 32046217 Free PMC article. Review.
Cited by
-
CRISPR/Cas-Based Prenatal Screening for Aneuploidy: Challenges and Opportunities for Early Diagnosis.Medicina (Kaunas). 2025 Mar 27;61(4):610. doi: 10.3390/medicina61040610. Medicina (Kaunas). 2025. PMID: 40282900 Free PMC article. Review.
-
Advancements in multi-omics research to address challenges in Alzheimer's disease: a systems biology approach utilizing molecular biomarkers and innovative strategies.Front Aging Neurosci. 2025 Jul 23;17:1591796. doi: 10.3389/fnagi.2025.1591796. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40771197 Free PMC article. Review.
-
Regulating cleavage activity and enabling microRNA detection with split sgRNA in Cas12b.Nat Commun. 2025 Jul 10;16(1):6392. doi: 10.1038/s41467-025-61748-4. Nat Commun. 2025. PMID: 40640155 Free PMC article.
-
Evolution of agricultural biotechnology is the paradigm shift in crop resilience and development: a review.Front Plant Sci. 2025 Jun 19;16:1585826. doi: 10.3389/fpls.2025.1585826. eCollection 2025. Front Plant Sci. 2025. PMID: 40612601 Free PMC article. Review.
References
-
- Kaminski M.M., Abudayyeh O.O., Gootenberg J.S., Zhang F., Collins J.J.. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021; 5:643–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

