Synergistic action of human RNaseH2 and the RNA helicase-nuclease DDX3X in processing R-loops
- PMID: 39189461
- PMCID: PMC11514492
- DOI: 10.1093/nar/gkae731
Synergistic action of human RNaseH2 and the RNA helicase-nuclease DDX3X in processing R-loops
Abstract
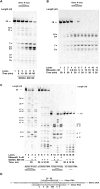
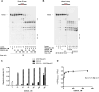
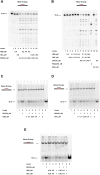
R-loops are three-stranded RNA-DNA hybrid structures that play important regulatory roles, but excessive or deregulated R-loops formation can trigger DNA damage and genome instability. Digestion of R-loops is mainly relying on the action of two specialized ribonucleases: RNaseH1 and RNaseH2. RNaseH2 is the main enzyme carrying out the removal of misincorporated rNMPs during DNA replication or repair, through the Ribonucleotide Excision Repair (RER) pathway. We have recently shown that the human RNA helicase DDX3X possessed RNaseH2-like activity, being able to substitute RNaseH2 in reconstituted RER reactions. Here, using synthetic R-loop mimicking substrates, we could show that human DDX3X alone was able to both displace and degrade the ssRNA strand hybridized to DNA. Moreover, DDX3X was found to physically interact with human RNaseH2. Such interaction suppressed the nuclease and helicase activities of DDX3X, but stimulated severalfold the catalytic activity of the trimeric RNaseH2, but not of RNaseH1. Finally, silencing of DDX3X in human cells caused accumulation of RNA-DNA hybrids and phosphorylated RPA foci. These results support a role of DDX3X as a scaffolding protein and auxiliary factor for RNaseH2 during R-loop degradation.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures







References
-
- Petereman E., Lam L., Zou L. Sources, resolution and physiological relevance of R-loops and RNA-DNA hybrids. Nat. Rev. Mol. Cell Biol. 2022; 23:521–540. - PubMed
-
- Lee D.Y., Clayton D.A.. Initiation of mitochondrial DNA replication by transcription and R-loop processing. J. Biol. Chem. 1998; 273:30614–30621. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

