Effects of postbiotics on chronic diarrhea in young adults: a randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles
- PMID: 39189588
- PMCID: PMC11352714
- DOI: 10.1080/19490976.2024.2395092
Effects of postbiotics on chronic diarrhea in young adults: a randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles
Abstract
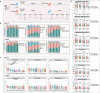
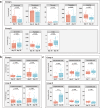
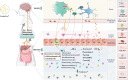
Chronic diarrhea has a considerable impact on quality of life. This randomized, double-blind, placebo-controlled crossover intervention trial was conducted with 69 participants (36 in Group A, 33 in Group B), aiming to investigate the potential of postbiotics in alleviating diarrhea-associated symptoms. Participants received postbiotic Probio-Eco® and placebo for 21 days each in alternating order, with a 14-day washout period between interventions. The results showed that postbiotic intake resulted in significant improvements in Bristol stool scale score, defecation frequency, urgency, and anxiety. Moreover, the postbiotic intervention increased beneficial intestinal bacteria, including Dysosmobacter welbionis and Faecalibacterium prausnitzii, while reducing potential pathogens like Megamonas funiformis. The levels of gut Microviridae notably increased. Non-targeted metabolomics analysis revealed postbiotic-driven enrichment of beneficial metabolites, including α-linolenic acid and p-methoxycinnamic acid, and reduction of diarrhea-associated metabolites, including theophylline, piperine, capsaicin, and phenylalanine. Targeted metabolomics confirmed a significant increase in fecal butyric acid after postbiotic intervention. The levels of aromatic amino acids, phenylalanine and tryptophan, and their related metabolites, 5-hydroxytryptophan and kynurenine, decreased after the postbiotic intervention, suggesting diarrhea alleviation was through modulating the tryptophan-5-hydroxytryptamine and tryptophan-kynurenine pathways. Additionally, chenodeoxycholic acid, a diarrhea-linked primary bile acid, decreased substantially. In conclusion, postbiotics have shown promise in relieving chronic diarrhea.
Keywords: Chronic diarrhea; gut metabolomics; gut microbiota; postbiotics; quality of life.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, Major G, O’Connor M, Sanders DS, Sinha R, et al. Guidelines for the investigation of chronic diarrhoea in adults: British society of gastroenterology, 3rd edition. Gut. 2018;67(8):1380–1399. doi: 10.1136/gutjnl-2017-315909. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
