Integration of genomic and pharmacokinetic data to predict clinical outcomes in HIV-associated cryptococcal meningitis
- PMID: 39189739
- PMCID: PMC11481554
- DOI: 10.1128/mbio.01592-24
Integration of genomic and pharmacokinetic data to predict clinical outcomes in HIV-associated cryptococcal meningitis
Abstract
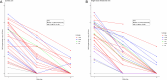
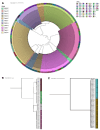
Cryptococcal meningitis causes an estimated 112,000 global deaths per annum. Genomic and phenotypic features of the infecting strain of Cryptococcus spp. have been associated with outcomes from cryptococcal meningitis. Additionally, population-level pharmacokinetic variability is well documented in these patient cohorts. The relative contribution of these factors to clinical outcomes is unknown. Based in Malawi, we conducted a sub-study of the phase 3 Ambition-CM trial (ISRCTN72509687), collecting plasma and cerebrospinal fluid at serial time points during the first 14 days of antifungal therapy. We explored the relative contribution of pathogen genotype, drug resistance phenotype, and pharmacokinetics on clinical outcomes including lumbar opening pressure, pharmacodynamic effect, and mortality. We report remarkable genomic homogeneity among infecting strains of Cryptococcus spp., within and between patients. There was no evidence of acquisition of antifungal resistance in our isolates. Genotypic features of the infecting strain were not consistently associated with adverse or favorable clinical outcomes. However, baseline fungal burden and early fungicidal activity (EFA) were associated with mortality. The strongest predictor of EFA was the level of exposure to amphotericin B. Our analysis suggests the most effective means of improving clinical outcomes from HIV-associated cryptococcal meningitis is to optimize exposure to potent antifungal therapy.
Importance: HIV-associated cryptococcal meningitis is associated with a high burden of mortality. Research into the different strain types causing this disease has yielded inconsistent findings in terms of which strains are associated with worse clinical outcomes. Our study suggests that the exposure of patients to potent anti-cryptococcal drugs has a more significant impact on clinical outcomes than the strain type of the infecting organism. Future research should focus on optimizing drug exposure, particularly in the context of novel anticryptococcal drugs coming into clinical use.
Keywords: Cryptococcus; cryptococcal meningitis; genomics; pharmacodynamics; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi:10.1093/cid/cit794 - DOI - PMC - PubMed
-
- Pullen MF, Hullsiek KH, Rhein J, Musubire AK, Tugume L, Nuwagira E, Abassi M, Ssebambulidde K, Mpoza E, Kiggundu R, Akampurira A, Nabeta HW, Schutz C, Evans EE, Rajasingham R, Skipper CP, Pastick KA, Williams DA, Morawski BM, Bangdiwala AS, Meintjes G, Muzoora C, Meya DB, Boulware DR. 2020. Cerebrospinal fluid early fungicidal activity as a surrogate endpoint for cryptococcal meningitis survival in clinical trials. Clin Infect Dis 71:e45–e49. doi:10.1093/cid/ciaa016 - DOI - PMC - PubMed
-
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarvis J, Bekker L-G, Wood R, Limmathurotsakul D, Chierakul W, Stepniewska K, White NJ, Jaffar S, Harrison TS. 2009. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 49:702–709. doi:10.1086/604716 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- R01 AI073896/AI/NIAID NIH HHS/United States
- R01 AI093257/AI/NIAID NIH HHS/United States
- 203919/Z/16/Z/Wellcome Trust (WT)
- National Institute for Health and Care Research (NIHR)
- AI73896/HHS | National Institutes of Health (NIH)
- AI93257/HHS | National Institutes of Health (NIH)
- NIHR134342/National Institute for Health and Care Research (NIHR)
- TRIA2015-1092/EC | H2020 | ERA-LEARN | European and Developing Countries Clinical Trials Partnership (EDCTP)
- RP-2017-08-ST2-012/National Institute for Health and Care Research (NIHR)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

