Discovery and Biosynthesis of Persiathiacins: Unusual Polyglycosylated Thiopeptides Active Against Multidrug Resistant Tuberculosis
- PMID: 39189814
- PMCID: PMC11406533
- DOI: 10.1021/acsinfecdis.4c00502
Discovery and Biosynthesis of Persiathiacins: Unusual Polyglycosylated Thiopeptides Active Against Multidrug Resistant Tuberculosis
Abstract
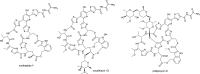
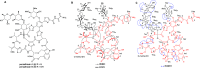
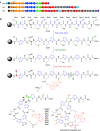
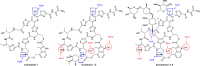
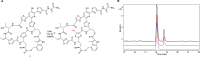
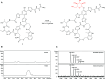
Thiopeptides are ribosomally biosynthesized and post-translationally modified peptides (RiPPs) that potently inhibit the growth of Gram-positive bacteria by targeting multiple steps in protein biosynthesis. The poor pharmacological properties of thiopeptides, particularly their low aqueous solubility, has hindered their development into clinically useful antibiotics. Antimicrobial activity screens of a library of Actinomycetota extracts led to discovery of the novel polyglycosylated thiopeptides persiathiacins A and B from Actinokineospora sp. UTMC 2448. Persiathiacin A is active against methicillin-resistant Staphylococcus aureus and several Mycobacterium tuberculosis strains, including drug-resistant and multidrug-resistant clinical isolates, and does not significantly affect the growth of ovarian cancer cells at concentrations up to 400 μM. Polyglycosylated thiopeptides are extremely rare and nothing is known about their biosynthesis. Sequencing and analysis of the Actinokineospora sp. UTMC 2448 genome enabled identification of the putative persiathiacin biosynthetic gene cluster (BGC). A cytochrome P450 encoded by this gene cluster catalyzes the hydroxylation of nosiheptide in vitro and in vivo, consistent with the proposal that the cluster directs persiathiacin biosynthesis. Several genes in the cluster encode homologues of enzymes known to catalyze the assembly and attachment of deoxysugars during the biosynthesis of other classes of glycosylated natural products. One of these encodes a glycosyl transferase that was shown to catalyze attachment of a D-glucose residue to nosiheptide in vitro. The discovery of the persiathiacins and their BGC thus provides the basis for the development of biosynthetic engineering approaches to the creation of novel (poly)glycosylated thiopeptide derivatives with enhanced pharmacological properties.
Keywords: 6-deoxysugar; RiPP; antibiotic; cytochrome P450; glycosyl transferase.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Global Tuberculosis Report; World Health Organization: Geneva, 2021.
-
- Treatment of Tuberculosis: Guidelines, 4th ed.; World Health Organization: Geneva, 2010. - PubMed
-
- Arnison P. G.; Bibb M. J.; Bierbaum G.; Bowers A. A.; Bugni T. S.; Bulaj G.; Camarero J. A.; Campopiano D. J.; Challis G. L.; Clardy J.; Cotter P. D.; Craik D. J.; Dawson M.; Dittmann E.; Donadio S.; Dorrestein P. C.; Entian K.-D.; Fischbach M. A.; Garavelli J. S.; Göransson U.; Gruber C. W.; Haft D. H.; Hemscheidt T. K.; Hertweck C.; Hill C.; Horswill A. R.; Jaspars M.; Kelly W. L.; Klinman J. P.; Kuipers O. P.; Link A. J.; Liu W.; Marahiel M. A.; Mitchell D. A.; Moll G. N.; Moore B. S.; Müller R.; Nair S. K.; Nes I. F.; Norris G. E.; Olivera B. M.; Onaka H.; Patchett M. L.; Piel J.; Reaney M. J. T.; Rebuffat S.; Ross R. P.; Sahl H.-G.; Schmidt E. W.; Selsted M. E.; Severinov K.; Shen B.; Sivonen K.; Smith L.; Stein T.; Süssmuth R. D.; Tagg J. R.; Tang G.-L.; Truman A. W.; Vederas J. C.; Walsh C. T.; Walton J. D.; Wenzel S. C.; Willey J. M.; van der Donk W. A. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30 (1), 108–160. 10.1039/C2NP20085F. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

