Androgen Receptor Inhibitors in Patients With Nonmetastatic Castration-Resistant Prostate Cancer
- PMID: 39190308
- PMCID: PMC11350483
- DOI: 10.1001/jamanetworkopen.2024.29783
Androgen Receptor Inhibitors in Patients With Nonmetastatic Castration-Resistant Prostate Cancer
Abstract
Importance: Novel androgen receptor inhibitors (ARIs; darolutamide, enzalutamide, and apalutamide) are standard-of-care treatments for nonmetastatic castration-resistant prostate cancer (nmCRPC). However, there are sparse data comparing their clinical use and tolerability.
Objective: To compare clinical use and outcomes for darolutamide, enzalutamide, and apalutamide in patients with nmCRPC.
Design, setting, and participants: This retrospective cohort study reviewed electronic medical records from the Precision Point Specialty network of US urology practices. Eligible patients had nmCRPC and no prior novel hormonal therapy and initiated novel ARI treatment between August 1, 2019, and March 31, 2022. Data were analyzed from February 1, 2019, to December 31, 2022.
Exposures: Patients were prescribed darolutamide, enzalutamide, or apalutamide as their first novel ARI for nmCRPC.
Main outcomes and measures: The main outcome was a composite of 2 end points, treatment discontinuation and progression to metastatic CRPC (mCRPC), whichever occurred first. Both end points were also assessed separately.
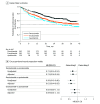
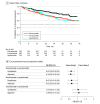
Results: All 870 patients meeting eligibility criteria were included (362 receiving darolutamide [41.6%]; 382, enzalutamide [43.9%]; 126, apalutamide [14.5%]); mean (SD) age was 78.8 (8.7) years. Self-reported race was Black or African American in 187 patients (21.5%), White in 585 (67.2%), and other or unknown in 98 (11.3%). The darolutamide cohort had lower proportions of patients with a composite end point event (134 [37.0%] vs 201 [52.6%] for enzalutamide and 66 [52.4%] for apalutamide), discontinuation (110 [30.4%] for darolutamide vs 156 [40.8%] for enzalutamide and 58 [46.0%] for apalutamide), and progression to mCRPC (64 [17.7%] for darolutamide vs 108 [28.3%] for enzalutamide and 35 [27.8%] for apalutamide) during the study period. After adjusting for baseline covariates, patients receiving darolutamide had a lower risk of a composite end point event compared with enzalutamide (risk reduction, 33.8%; hazard ratio [HR], 0.66 [95% CI, 0.53-0.84]) and apalutamide (risk reduction, 35.1%; HR, 0.65 [95% CI, 0.48-0.88]). Similarly, patients receiving darolutamide had a lower risk of discontinuation compared with enzalutamide (risk reduction, 27.4%; HR, 0.73 [95% CI, 0.56-0.94]) and apalutamide (risk reduction, 39.1%; HR, 0.61 [95% CI, 0.44-0.85]) and a lower risk of progression to mCRPC compared with enzalutamide (risk reduction, 40.6%; HR, 0.59 [95% CI, 0.43-0.82]) and apalutamide (risk reduction, 35.3%; HR, 0.65 [95% CI, 0.42-0.99]). There was no difference between enzalutamide and apalutamide treatment across outcomes.
Conclusions and relevance: In this large cohort study of patients with nmCRPC treated with novel ARIs, results suggest better tolerability for darolutamide compared with enzalutamide and apalutamide, which may be associated with a clinical effectiveness advantage. Comparative clinical studies are needed to guide treatment decisions in the absence of head-to-head clinical trials.
Conflict of interest statement
Figures


References
-
- Schaeffer EM, Srinivas S, Adra N, et al. . NCCN Guidelines Insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298. - PubMed
-
- Gillessen S, Bossi A, Davis ID, et al. . Management of patients with advanced prostate cancer—metastatic and/or castration-resistant prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185:178-215. doi:10.1016/j.ejca.2023.02.018 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

