Microglial cGAS-STING signaling underlies glaucoma pathogenesis
- PMID: 39190350
- PMCID: PMC11388346
- DOI: 10.1073/pnas.2409493121
Microglial cGAS-STING signaling underlies glaucoma pathogenesis
Abstract
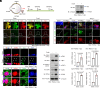
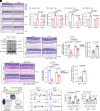
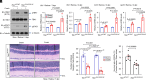
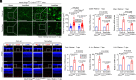
Characterized by progressive degeneration of retinal ganglion cells (RGCs) and vision loss, glaucoma is the primary cause of irreversible blindness, incurable and affecting over 78 million patients. However, pathogenic mechanisms leading to glaucoma-induced RGC loss are incompletely understood. Unexpectedly, we found that cGAS-STING (2'3'-cyclic GMP-AMP-stimulator of interferon genes) signaling, which surveils displaced double-stranded DNA (dsDNA) in the cytosol and initiates innate immune responses, was robustly activated during glaucoma in retinal microglia in distinct murine models. Global or microglial deletion of STING markedly relieved glaucoma symptoms and protected RGC degeneration and vision loss, while mice bearing genetic cGAS-STING supersensitivity aggravated retinal neuroinflammation and RGC loss. Mechanistically, dsDNA from tissue injury activated microglial cGAS-STING signaling, causing deleterious macroglia reactivity in retinas by cytokine-mediated microglia-macroglia interactions, progressively driving apoptotic death of RGCs. Remarkably, preclinical investigations of targeting cGAS-STING signaling by intraocular injection of TBK1i or anti-IFNAR1 antibody prevented glaucoma-induced losses of RGCs and vision. Therefore, we unravel an essential role of cGAS-STING signaling underlying glaucoma pathogenesis and suggest promising therapeutic strategies for treating this devastating disease.
Keywords: cGAS–STING; glaucoma; microglia; neuroinflammation; vision loss.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

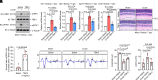
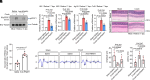

References
-
- Jonas J. B., et al. , Glaucoma. Lancet 390, 2183–2193 (2017). - PubMed
-
- Yu D. Y., et al. , Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog. Retin. Eye Res. 36, 217–246 (2013). - PubMed
-
- Krauss R., Bosanac T., Devraj R., Engber T., Hughes R. O., Axons matter: The promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol. Sci. 41, 281–293 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFA1301401/MOST | National Key Research and Development Program of China (NKPs)
- 32321002/MOST | National Natural Science Foundation of China (NSFC)
- 31830052/MOST | National Natural Science Foundation of China (NSFC)
- 82371870/MOST | National Natural Science Foundation of China (NSFC)
- 81902915/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

