miR-33 deletion in hepatocytes attenuates MASLD-MASH-HCC progression
- PMID: 39190492
- PMCID: PMC11466198
- DOI: 10.1172/jci.insight.168476
miR-33 deletion in hepatocytes attenuates MASLD-MASH-HCC progression
Abstract
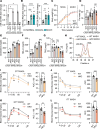
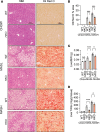
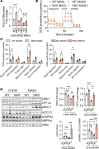
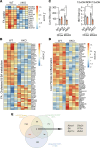
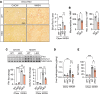
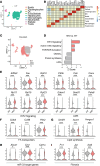
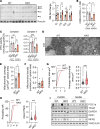
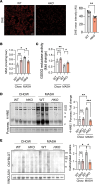
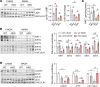
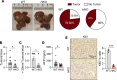
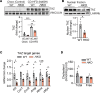
The complexity of the mechanisms underlying metabolic dysfunction-associated steatotic liver disease (MASLD) progression remains a significant challenge for the development of effective therapeutics. miRNAs have shown great promise as regulators of biological processes and as therapeutic targets for complex diseases. Here, we study the role of hepatic miR-33, an important regulator of lipid metabolism, during the progression of MASLD and the development of hepatocellular carcinoma (HCC). We report that miR-33 was elevated in the livers of humans and mice with MASLD and that its deletion in hepatocytes (miR-33 HKO) improved multiple aspects of the disease, including steatosis and inflammation, limiting the progression to metabolic dysfunction-associated steatotic hepatitis (MASH), fibrosis, and HCC. Mechanistically, hepatic miR-33 deletion reduced lipid synthesis and promoted mitochondrial fatty acid oxidation, reducing lipid burden. Additionally, absence of miR-33 altered the expression of several known miR-33 target genes involved in metabolism and resulted in improved mitochondrial function and reduced oxidative stress. The reduction in lipid accumulation and liver injury resulted in decreased YAP/TAZ pathway activation, which may be involved in the reduced HCC progression in HKO livers. Together, these results suggest suppressing hepatic miR-33 may be an effective therapeutic approach to temper the development of MASLD, MASH, and HCC in obesity.
Keywords: Fatty acid oxidation; Hepatology; Liver cancer; Metabolism; Noncoding RNAs.
Conflict of interest statement
Figures


Update of
-
Hepatocyte-specific miR-33 deletion attenuates NAFLD-NASH-HCC progression.bioRxiv [Preprint]. 2023 Jan 20:2023.01.18.523503. doi: 10.1101/2023.01.18.523503. bioRxiv. 2023. Update in: JCI Insight. 2024 Aug 27;9(19):e168476. doi: 10.1172/jci.insight.168476. PMID: 36711578 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

