The senescence-associated secretome of Hedgehog-deficient hepatocytes drives MASLD progression
- PMID: 39190624
- PMCID: PMC11444248
- DOI: 10.1172/JCI180310
The senescence-associated secretome of Hedgehog-deficient hepatocytes drives MASLD progression
Abstract
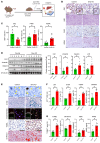
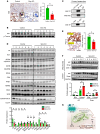
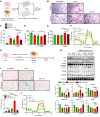
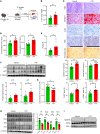
The burden of senescent hepatocytes correlates with the severity of metabolic dysfunction-associated steatotic liver disease (MASLD), but the mechanisms driving senescence and how it exacerbates MASLD are poorly understood. Hepatocytes experience lipotoxicity and become senescent when Smoothened (Smo) is deleted to disrupt Hedgehog signaling. We aimed to determine whether the secretomes of Smo-deficient hepatocytes perpetuate senescence to drive MASLD progression. RNA-Seq analysis of liver samples from human and murine cohorts with MASLD confirmed that hepatocyte populations in MASLD livers were depleted of Smo+ cells and enriched with senescent cells. When fed a choline-deficient, amino acid-restricted high-fat diet (CDA-HFD) to induce MASLD, Smo- mice had lower antioxidant markers and developed worse DNA damage, senescence, steatohepatitis, and fibrosis than did Smo+ mice. Sera and hepatocyte-conditioned medium from Smo- mice were depleted of thymidine phosphorylase (TP), a protein that maintains mitochondrial fitness. Treating Smo- hepatocytes with TP reduced senescence and lipotoxicity, whereas inhibiting TP in Smo+ hepatocytes had the opposite effect and exacerbated hepatocyte senescence, steatohepatitis, and fibrosis in CDA-HFD-fed mice. We conclude that inhibition of Hedgehog signaling in hepatocytes promoted MASLD by suppressing hepatocyte production of proteins that prevent lipotoxicity and senescence.
Keywords: Cell stress; Cellular senescence; Hepatology; Metabolism; Mitochondria.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

