A functional genomic framework to elucidate novel causal metabolic dysfunction-associated fatty liver disease genes
- PMID: 39190705
- PMCID: PMC11865357
- DOI: 10.1097/HEP.0000000000001066
A functional genomic framework to elucidate novel causal metabolic dysfunction-associated fatty liver disease genes
Abstract
Background and aims: Metabolic dysfunction-associated fatty liver disease (MASLD) is the most prevalent chronic liver pathology in western countries, with serious public health consequences. Efforts to identify causal genes for MASLD have been hampered by the relative paucity of human data from gold standard magnetic resonance quantification of hepatic fat. To overcome insufficient sample size, genome-wide association studies using MASLD surrogate phenotypes have been used, but only a small number of loci have been identified to date. In this study, we combined genome-wide association studies of MASLD composite surrogate phenotypes with genetic colocalization studies followed by functional in vitro screens to identify bona fide causal genes for MASLD.
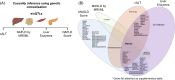
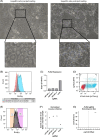
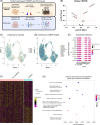
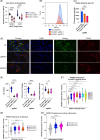
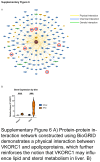
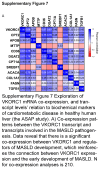
Approach and results: We used the UK Biobank to explore the associations of our novel MASLD score, and genetic colocalization to prioritize putative causal genes for in vitro validation. We created a functional genomic framework to study MASLD genes in vitro using CRISPRi. Our data identify VKORC1 , TNKS , LYPLAL1 , and GPAM as regulators of lipid accumulation in hepatocytes and suggest the involvement of VKORC1 in the lipid storage related to the development of MASLD.
Conclusions: Complementary genetic and genomic approaches are useful for the identification of MASLD genes. Our data supports VKORC1 as a bona fide MASLD gene. We have established a functional genomic framework to study at scale putative novel MASLD genes from human genetic association studies.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Johanne M. Justesen is employed by Novo Nordisk. Joshua W. Knowles consults for Arrowhead and Mammoth. The remaining authors have no conflicts to report.
Figures









Update of
-
A functional genomic framework to elucidate novel causal non-alcoholic fatty liver disease genes.medRxiv [Preprint]. 2024 Feb 4:2024.02.03.24302258. doi: 10.1101/2024.02.03.24302258. medRxiv. 2024. Update in: Hepatology. 2025 Jul 1;82(1):165-183. doi: 10.1097/HEP.0000000000001066. PMID: 38352379 Free PMC article. Updated. Preprint.
References
-
- Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168–85. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

