Interictal blood-brain barrier dysfunction in piriform cortex of people with epilepsy
- PMID: 39190772
- PMCID: PMC11514923
- DOI: 10.1002/acn3.52176
Interictal blood-brain barrier dysfunction in piriform cortex of people with epilepsy
Abstract
Objective: The piriform cortex is considered to be highly epileptogenic. Its resection during epilepsy surgery is a predictor for postoperative seizure freedom in temporal lobe epilepsy. Epilepsy is associated with a dysfunction of the blood-brain barrier. We investigated blood-brain barrier dysfunction in the piriform cortex of people with temporal lobe epilepsy using quantitative T1-relaxometry.
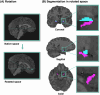
Methods: Gadolinium-based contrast agent was administered ictally and interictally in 37 individuals before undergoing quantitative T1-relaxometry. Postictal and interictal images were co-registered, and subtraction maps were created as biomarkers for peri-ictal (∆qT1interictal-postictal) and interictal (∆qT1noncontrast-interictal) blood-brain barrier dysfunction. Values were extracted for the piriform cortex, hippocampus, amygdala, and the whole cortex.
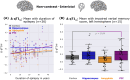
Results: In temporal lobe epilepsy (n = 14), ∆qT1noncontrast-interictal was significantly higher in the piriform cortex than in the whole cortex (p = 0.02). In extratemporal lobe epilepsy (n = 23), ∆qT1noncontrast-interictal was higher in the hippocampus than in the whole cortex (p = 0.05). Across all individuals (n = 37), duration of epilepsy was correlated with ∆qT1noncontrast-interictal (ß = 0.001, p < 0.001) in all regions, while the association was strongest in the piriform cortex. Impaired verbal memory was associated with ∆qT1noncontrast-interictal only in the piriform cortex (p = 0.04). ∆qT1interictal-postictal did not show differences in any region.
Interpretation: Interictal blood-brain barrier dysfunction occurs in the piriform cortex in temporal lobe epilepsy. This dysfunction is linked to longer disease duration and worse cognitive deficits, emphasizing the central role of the piriform cortex in the epileptogenic network of temporal lobe epilepsy.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Juri‐Alexander Witt reports personal fees from Eisai. These activities were not related to the content of this manuscript. Randi von Wrede has received travel support, fees as speaker or for serving on the advisory board from Angelini, Apocare, Arvelle, Cerbomed, Desitin, Eisai, GW pharmaceuticals‐JAZZ pharma, and UCB Pharma. These activities were not related to the content of this manuscript. Valeri Borger has received fees for serving as clinical consultant from Brainlab AG. These activities were not related to the content of this manuscript. Christoph Helmstaedter has received grants from the European Union, travel support from Desitin, honoraria for lectures, counseling, and advisory boards from GW Pharmaceuticals, EISAI, and UCB, as well as license fees from EISAI and UCB. Rainer Surges has received fees as speaker or for serving on the advisory board from Angelini, Arvelle, Bial, Desitin, Eisai, Janssen‐Cilag GmbH, LivaNova, Novartis, Precisis GmbH, UCB Pharma, UnEEG, and Zogenix and grants from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF), the Bundesministerium für Gesundheit, and the Marga and Walter Boll Stiftung. These activities were not related to the content of this manuscript. Theodor Rüber declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The other authors report no competing interests.
Figures

References
-
- Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300(21):2497‐2505. - PubMed
-
- Bar‐Klein G, Lublinsky S, Kamintsky L, et al. Imaging blood‐brain barrier dysfunction as a biomarker for epileptogenesis. Brain J Neurol. 2017;140(6):1692‐1705. - PubMed
-
- Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317(6038):623‐625. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

