CD94+ natural killer cells potentiate pulmonary ischaemia-reperfusion injury
- PMID: 39190789
- PMCID: PMC12128193
- DOI: 10.1183/13993003.02171-2023
CD94+ natural killer cells potentiate pulmonary ischaemia-reperfusion injury
Abstract
Background: Pulmonary ischaemia-reperfusion injury (IRI) is a major contributor to poor lung transplant outcomes. We recently demonstrated a central role of airway-centred natural killer (NK) cells in mediating IRI; however, there are no existing effective therapies for directly targeting NK cells in humans.
Methods: We hypothesised that a depleting anti-CD94 monoclonal antibody (mAb) would provide therapeutic benefit in mouse and human models of IRI based on high levels of KLRD1 (CD94) transcripts in bronchoalveolar lavage samples from lung transplant patients.
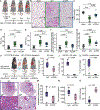
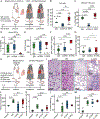
Results: We found that CD94 is highly expressed on mouse and human NK cells, with increased expression during IRI. Anti-mouse and anti-human mAbs against CD94 showed effective NK cell depletion in mouse and human models and blunted lung damage and airway epithelial killing, respectively. In two different allogeneic orthotopic lung transplant mouse models, anti-CD94 treatment during induction reduced early lung injury and chronic inflammation relative to control therapies. Anti-CD94 did not increase donor antigen-presenting cells that could alter long-term graft acceptance.
Conclusions: Lung transplant induction regimens incorporating anti-CD94 treatment may safely improve early clinical outcomes.
Copyright ©The authors 2024. For reproduction rights and permissions contact permissions@ersnet.org.
Conflict of interest statement
Conflict of interest: R. Shi, L. Bai, K. Dilley, M. Will, N. Tomasevic, T. Sputova, A. Salles and J. Kang are employees and shareholders of Dren Bio, Inc. N. Tomasevic is a founder and holds a management position at Dren Bio, Inc. M. Will holds a management position at Dren Bio, Inc. L.L. Lanier is a consultant for Dren Bio, Inc. The authors have no additional conflicts of interest to disclose.
Figures


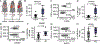


References
-
- Snell GI, Yusen RD, Weill D et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading; A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36(10):1097–1103. - PubMed
-
- Kolaitis NA, Gao Y, Soong A et al. Primary graft dysfunction attenuates improvements in health-related quality of life after lung transplantation, but not disability or depression. Am J Transplant 2021;21(2):815–824. - PubMed
-
- Paraskeva MA, Borg BM, Paul E et al. Abnormal one-year post-lung transplant spirometry is a significant predictor of increased mortality and chronic lung allograft dysfunction. J Heart Lung Transplant 2021;40(12):1649–1657. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
