Evaluation of C-Reactive Protein and Computer-Aided Analysis of Chest X-rays as Tuberculosis Triage Tests at Health Facilities in Lesotho and South Africa
- PMID: 39190813
- PMCID: PMC11581699
- DOI: 10.1093/cid/ciae378
Evaluation of C-Reactive Protein and Computer-Aided Analysis of Chest X-rays as Tuberculosis Triage Tests at Health Facilities in Lesotho and South Africa
Abstract
Background: To improve tuberculosis case-finding, rapid, non-sputum triage tests need to be developed according to the World Health Organization target product profile (TPP) (>90% sensitivity, >70% specificity). We prospectively evaluated and compared artificial intelligence-based, computer-aided detection software, CAD4TBv7, and C-reactive protein assay (CRP) as triage tests at health facilities in Lesotho and South Africa.
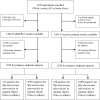
Methods: Adults (≥18 years) presenting with ≥1 of the 4 cardinal tuberculosis symptoms were consecutively recruited between February 2021 and April 2022. After informed consent, each participant underwent a digital chest X-ray for CAD4TBv7 and a CRP test. Participants provided 1 sputum sample for Xpert MTB/RIF Ultra and Xpert MTB/RIF and 1 for liquid culture. Additionally, an expert radiologist read the chest X-rays via teleradiology. For primary analysis, a composite microbiological reference standard (ie, positive culture or Xpert Ultra) was used.
Results: We enrolled 1392 participants, 48% were people with HIV and 24% had previously tuberculosis. The receiver operating characteristic curve for CAD4TBv7 and CRP showed an area under the curve of .87 (95% CI: .84-.91) and .80 (95% CI: .76-.84), respectively. At thresholds corresponding to 90% sensitivity, specificity was 68.2% (95% CI: 65.4-71.0%) and 38.2% (95% CI: 35.3-41.1%) for CAD4TBv7 and CRP, respectively. CAD4TBv7 detected tuberculosis as well as an expert radiologist. CAD4TBv7 almost met the TPP criteria for tuberculosis triage.
Conclusions: CAD4TBv7 is accurate as a triage test for patients with tuberculosis symptoms from areas with a high tuberculosis and HIV burden. The role of CRP in tuberculosis triage requires further research.
Clinical trials registration: Clinicaltrials.gov identifier: NCT04666311.
Keywords: C-reactive protein; chest X-ray; computer-aided detection; triage test; tuberculosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. B. v. G. reports that his group received royalties from Delft Imaging Systems and that he holds shares in Thirona. K. R., B. v. G., A. v. H., A. S., K. M., L. L., T. Z., B. K. M. J., S. B., T. M., and M. K. report support related to this work from the European and Developing Countries Clinical Trials Partnership 2 (EDCTP2). K. R. reports grants unrelated to this work from EDCTP2 and Horizon 2020, as well as unpaid participation on a data safety monitoring board or scientific advisory board for evaluation of new TB diagnostics. S. B. reports a grant unrelated to this work from the National Institutes of Health (NIH) and travel support from MSD. T. M. reports travel support from MSD. N. D. L. reports support for travel from ViiV Healthcare and Gilead Sciences Sarl as well as for attending a board meeting from ViiV Healthcare and Pharming. S. B. and T. M. report travel support from MSD. The other authors report no potential conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- World Health Organization . Global tuberculosis report 2022. Geneva, Switzerland; World Health Organization, 2022.
-
- World Health Organization . High-priority target product profiles for new tuberculosis diagnostics. Geneva, Switzerland; World Health Organization, 2014.
-
- World Health Organization . WHO consolidated guidelines on tuberculosis. Module 2: screening—systematic screening for tuberculosis disease. Geneva, Switzerland; World Health Organization, 2021. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

