Neurosurgical gene therapy for central nervous system diseases
- PMID: 39191071
- PMCID: PMC11445594
- DOI: 10.1016/j.neurot.2024.e00434
Neurosurgical gene therapy for central nervous system diseases
Abstract
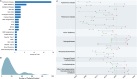
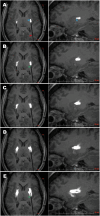
Viral vector mediated gene therapies for neurodegenerative and neurodevelopmental conditions that require neurosurgical administration continue to expand. We systematically reviewed the National Institutes of Health (NIH) ClinicalTrials.gov database to identify all clinical trials studying in-vivo viral vector mediated gene therapies targeted to the CNS for neurodegenerative and neurodevelopmental diseases. We isolated studies which delivered therapies using neurosurgical approaches: intracisternal, intraventricular, and/or intraparenchymal. Clinical trials primarily registered in international countries were included if they were referenced by an NIH registered clinical trial. We performed a scoping review to identify the preclinical studies that supported each human clinical trial. Key preclinical and clinical data were aggregated to characterize vector capsid design, delivery methods, gene expression profile, and clinical benefit. A total of 64 clinical trials were identified in active, completed, terminated, and long-term follow-up stages. A range of CNS conditions across pediatric and adult populations are being studied with CNS targeted viral vector gene therapy, including Alzheimer's disease, Parkinson's disease, AADC deficiency, sphingolipidoses, mucopolysaccharidoses, neuronal ceroid lipofuscinoses, spinal muscular atrophy, adrenoleukodystrophy, Canavan disease, frontotemporal dementia, Huntington's disease, Rett syndrome, Dravet syndrome, mesial temporal lobe epilepsy, and glutaric acidemia. Adeno-associated viral vectors (AAVs) were utilized by the majority of tested therapies, with vector serotypes, regulatory elements, delivery methods, and vector monitoring varying based on the disease being studied. Intraparenchymal delivery has evolved significantly, with MRI-guided convection-enhanced delivery established as a gold standard method for pioneering novel gene targets.
Keywords: Clinical trials; Gene therapy; Gene transfer; Neurological disorders; Viral vectors.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mark Richardson reports a relationship with uniQure biopharma BV that includes: consulting or advisory. Mark Richardson reports a relationship with AviadoBio that includes: consulting or advisory. Mark Richardson reports a relationship with ClearPoint Neuro that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

