Association of early blood-based biomarkers and six-month functional outcomes in conventional severity categories of traumatic brain injury: capturing the continuous spectrum of injury
- PMID: 39191173
- PMCID: PMC11400615
- DOI: 10.1016/j.ebiom.2024.105298
Association of early blood-based biomarkers and six-month functional outcomes in conventional severity categories of traumatic brain injury: capturing the continuous spectrum of injury
Abstract
Background: Traumatic brain injury is conventionally categorised as mild, moderate, or severe on the Glasgow Coma Scale (GCS). Recently developed biomarkers can provide more objective and nuanced measures of the extent of brain injury.
Methods: Exposure-response relationships were investigated in 2479 patients aged ≥16 enrolled in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) prospective observational cohort study. Neurofilament protein-light (NFL), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), and glial fibrillary acidic protein (GFAP) were assayed from serum sampled in the first 24 h; concentrations were divided into quintiles within GCS severity groups. Relationships with the Glasgow Outcome Scale-Extended were examined using modified Poisson regression including age, sex, major extracranial injury, time to sample, and log biomarker concentration as covariates.
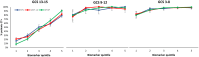
Findings: Within severity groups there were associations between biomarkers and outcomes after adjustment for covariates: GCS 13-15 and negative CT imaging (relative risks [RRs] from 1.28 to 3.72), GCS 13-15 and positive CT (1.21-2.81), GCS 9-12 (1.16-2.02), GCS 3-8 (1.09-1.94). RRs were associated with clinically important differences in expectations of prognosis. In patients with GCS 3 (RRs 1.51-1.80) percentages of unfavourable outcome were 37-51% in the lowest quintiles of biomarker levels and reached 90-94% in the highest quintiles. Similarly, for GCS 15 (RRs 1.83-3.79), the percentages were 2-4% and 19-28% in the lowest and highest biomarker quintiles, respectively.
Interpretation: Conventional TBI severity classification is inadequate and underestimates heterogeneity of brain injury and associated outcomes. The adoption of circulating biomarkers can add to clinical assessment of injury severity.
Funding: European Union 7th Framework program (EC grant 602150), Hannelore Kohl Stiftung, One Mind, Integra LifeSciences, Neuro-Trauma Sciences, NIHR Rosetrees Trust.
Keywords: Blood biomarkers; GFAP; NFL; Outcomes; Traumatic brain injury; UCH-L1.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Data were obtained in CENTER-TBI, a large collaborative project with the support of the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA) and from Integra LifeSciences Corporation (USA), and NeuroTrauma Sciences (USA). Data for the CENTER-TBI study has been collected through the Quesgen e-CRF (Quesgen Systems Inc, USA), hosted on the INCF platform and extracted via the INCF Neurobot tool (INCF, Sweden). VFJN holds a NIHR Rosetrees Trust Advanced Fellowship, NIHR302544, which is funded in partnership by the NIHR and Rosetrees Trust. DKM reports grants, personal fees, and non-financial support from GlaxoSmithKline outside the submitted work; personal fees from Neurotrauma Sciences, Lantmannen AB, PressuraNeuro, CSL Behring, and Invex Ltd, outside of the submitted work. Dr Maas reports receiving personal fees from NeuroTrauma Sciences, and PressuraNeuro outside the submitted work. VFJN reports a grant with ROCHE Pharmaceuticals outside the submitted work, and personal fees from Integra outside the submitted work. Dr Wilson reports receiving personal fees from Novartis, Neurotrauma Sciences, and Mass General Brigham outside the submitted work. No other disclosures were reported.
Figures


References
-
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–84. - PubMed
-
- Carroll L.J., Cassidy J.D., Peloso P.M., et al. Prognosis for mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:84–105. - PubMed
-
- Cancelliere C., Verville L., Stubbs J.L., et al. Post-concussion symptoms and disability in adults with mild traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2023;40(11-12):1045–1059. - PubMed
-
- National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Health Care Services. Board on Health Sciences Policy. Committee on Accelerating Progress in Traumatic Brain Injury Research and Care . In: Traumatic brain injury: a roadmap for accelerating progress. Matney C., Bowman K., Berwick D., editors. National Academies Press (US); Washington (DC): 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

