A Mitochondria-Targeting SIRT3 Inhibitor with Activity against Diffuse Large B Cell Lymphoma
- PMID: 39191393
- PMCID: PMC11403614
- DOI: 10.1021/acs.jmedchem.4c01053
A Mitochondria-Targeting SIRT3 Inhibitor with Activity against Diffuse Large B Cell Lymphoma
Abstract
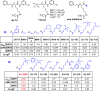
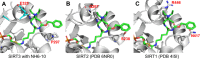
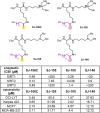
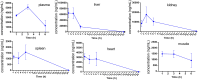
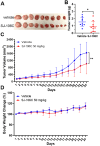
Diffuse large B-cell lymphomas (DLBCLs) are heterogeneous cancers that still require better and less toxic treatments. SIRT3, a member of the sirtuin family of NAD+-dependent protein deacylase, is critical for DLBCL growth and survival. A mitochondria-targeted SIRT3 small-molecule inhibitor, YC8-02, exhibits promising activity against DLBCL. However, YC8-02 has several limitations including poor solubility. Here, we report our medicinal chemistry efforts that led to an improved mitochondria-targeted SIRT3 inhibitor, SJ-106C, achieved by using a triethylammonium group, which helps to increase both solubility and SIRT3 inhibition potency. SJ-106C, while still inhibiting SIRT1 and SIRT2, is enriched in the mitochondria to help with SIRT3 inhibition. It is more active against DLBCL than other solid tumor cells and effectively inhibits DLBCL xenograft tumor growth. The findings provide useful insights for the development of SIRT3 inhibitors and mitochondrial targeting agents and further support the notion that SIRT3 is a promising druggable target for DLBCL.
Conflict of interest statement
The authors declare the following competing financial interest(s): HL is a founder and consultant for Sedec Therapeutics.
Figures



References
-
- Jiang Y.; Redmond D.; Nie K.; Eng K. W.; Clozel T.; Martin P.; Tan L. H.; Melnick A. M.; Tam W.; Elemento O. Deep sequencing reveals clonal evolution patterns and mutation events associated with relapse in B-cell lymphomas. Genome Biol. 2014, 15, 432. 10.1186/PREACCEPT-6612547881370092. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

