Reprogramming of breast tumor-associated macrophages with modulation of arginine metabolism
- PMID: 39191486
- PMCID: PMC11350068
- DOI: 10.26508/lsa.202302339
Reprogramming of breast tumor-associated macrophages with modulation of arginine metabolism
Abstract
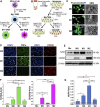
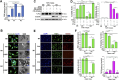
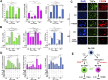
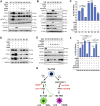
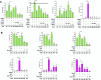
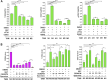
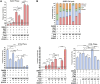
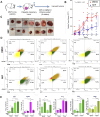
HER2+ breast tumors have abundant immune-suppressive cells, including M2-type tumor-associated macrophages (TAMs). Although TAMs consist of the immune-stimulatory M1 type and immune-suppressive M2 type, the M1/M2-TAM ratio is reduced in immune-suppressive tumors, contributing to their immunotherapy refractoriness. M1- versus M2-TAM formation depends on differential arginine metabolism, where M1-TAMs convert arginine to nitric oxide (NO) and M2-TAMs convert arginine to polyamines (PAs). We hypothesize that such distinct arginine metabolism in M1- versus M2-TAMs is attributed to different availability of BH4 (NO synthase cofactor) and that its replenishment would reprogram M2-TAMs to M1-TAMs. Recently, we reported that sepiapterin (SEP), the endogenous BH4 precursor, elevates the expression of M1-TAM markers within HER2+ tumors. Here, we show that SEP restores BH4 levels in M2-like macrophages, which then redirects arginine metabolism to NO synthesis and converts M2 type to M1 type. The reprogrammed macrophages exhibit full-fledged capabilities of antigen presentation and induction of effector T cells to trigger immunogenic cell death of HER2+ cancer cells. This study substantiates the utility of SEP in the metabolic shift of the HER2+ breast tumor microenvironment as a novel immunotherapeutic strategy.
© 2024 Fernando et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Update of
-
Reprogramming of breast tumor-associated macrophages with modulation of arginine metabolism.bioRxiv [Preprint]. 2023 Aug 22:2023.08.22.554238. doi: 10.1101/2023.08.22.554238. bioRxiv. 2023. Update in: Life Sci Alliance. 2024 Aug 27;7(11):e202302339. doi: 10.26508/lsa.202302339. PMID: 37662241 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
