Hyperuricemia and its related diseases: mechanisms and advances in therapy
- PMID: 39191722
- PMCID: PMC11350024
- DOI: 10.1038/s41392-024-01916-y
Hyperuricemia and its related diseases: mechanisms and advances in therapy
Abstract
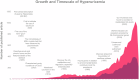
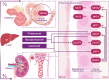
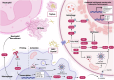
Hyperuricemia, characterized by elevated levels of serum uric acid (SUA), is linked to a spectrum of commodities such as gout, cardiovascular diseases, renal disorders, metabolic syndrome, and diabetes, etc. Significantly impairing the quality of life for those affected, the prevalence of hyperuricemia is an upward trend globally, especially in most developed countries. UA possesses a multifaceted role, such as antioxidant, pro-oxidative, pro-inflammatory, nitric oxide modulating, anti-aging, and immune effects, which are significant in both physiological and pathological contexts. The equilibrium of circulating urate levels hinges on the interplay between production and excretion, a delicate balance orchestrated by urate transporter functions across various epithelial tissues and cell types. While existing research has identified hyperuricemia involvement in numerous biological processes and signaling pathways, the precise mechanisms connecting elevated UA levels to disease etiology remain to be fully elucidated. In addition, the influence of genetic susceptibilities and environmental determinants on hyperuricemia calls for a detailed and nuanced examination. This review compiles data from global epidemiological studies and clinical practices, exploring the physiological processes and the genetic foundations of urate transporters in depth. Furthermore, we uncover the complex mechanisms by which the UA induced inflammation influences metabolic processes in individuals with hyperuricemia and the association with its relative disease, offering a foundation for innovative therapeutic approaches and advanced pharmacological strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
- 82002339, 81820108020/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

