Healthy Eating and Active Lifestyle after Bowel Cancer (HEAL ABC)-feasibility randomised controlled trial
- PMID: 39191956
- PMCID: PMC11611738
- DOI: 10.1038/s41430-024-01491-z
Healthy Eating and Active Lifestyle after Bowel Cancer (HEAL ABC)-feasibility randomised controlled trial
Abstract
Background: Evidence from cohort studies indicates that a healthy lifestyle can improve cancer survival but evidence from randomised controlled trials (RCT) is lacking. Thus, this study tested the feasibility of conducting a lifestyle intervention in patients after colorectal cancer (CRC) treatment.
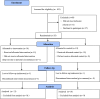
Methods: An intervention was developed based on World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) recommendations, the Health Action Process Approach, Motivational Interviewing and tested a feasibility, mixed-methods RCT. Participants were allocated to a three-month telephone-based intervention versus standard care control group. The follow up period was six months. Data on feasibility and secondary outcomes were collected and analysed using Stata (V15, StataCorp LLC) and NVivo 12 (QSR International Pty Ltd., Doncaster, VIC).
Results: Recruitment was challenging (31 ineligible, 37 declined; recruitment rate = 48.6%.). In total, 34/35 participants completed the intervention, and 31 (89%) completed follow up; all 31 completers participated in six telephone calls during intervention and six months follow up. Study retention was 97% (34/35) and 89% (31/35) at three and six months, respectively. Data completion rates were high (>90%). Intervention was acceptable to participants, met their needs and kept them accountable towards their goals. Participants in the intervention group showed significant improvement in WCRF/AICR, Diet Quality Index-International score and a 10% reduction in ultra-processed food consumption.
Conclusions: The HEAL ABC intervention was feasible for 87% of intervention participants, supporting them in healthy lifestyle changes. However, alternative recruitment strategies are needed for a fully powered RCT to determine the effectiveness of the intervention.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: The study was approved by the North West Greater Manchester South Research Ethics Committee (IRAS ID 273818), and registered on the National Institute of Health Clinical Trials register (NCT04227353).
Figures
References
-
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–44. - PubMed
-
- Firkins J, Hansen L, Driessnack M, Dieckmann N. Quality of life in “chronic” cancer survivors: a meta-analysis. J Cancer Surviv. 2020;14:504–17. - PubMed
-
- Vergnaud AC, Romaguera D, Peeters PH, van Gils CH, Chan DS, Romieu I, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study. Am J Clin Nutr. 2013;97:1107–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous