Prenatal vitamin D deficiency exposure leads to long-term changes in immune cell proportions
- PMID: 39191975
- PMCID: PMC11349904
- DOI: 10.1038/s41598-024-70911-8
Prenatal vitamin D deficiency exposure leads to long-term changes in immune cell proportions
Abstract
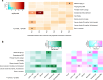
Vitamin D deficiency is a common deficiency worldwide, particularly among women of reproductive age. During pregnancy, it increases the risk of immune-related diseases in offspring later in life. However, how the body remembers exposure to an adverse environment during development is poorly understood. Herein, we explore the effects of prenatal vitamin D deficiency on immune cell proportions in offspring using vitamin D deficient mice established by dietary manipulation. We found that prenatal vitamin D deficiency alters immune cell proportions in offspring by changing the transcriptional properties of genes downstream of vitamin D receptor signaling in hematopoietic stem and progenitor cells of both the fetus and adults. Moreover, further investigations of the associations between maternal vitamin D levels and cord blood immune cell profiles from 75 healthy pregnant women and their term offspring also confirm that maternal vitamin D levels in the second trimester significantly affect immune cell proportions in the offspring. These findings imply that the differentiation properties of hematopoiesis act as long-term memories of prenatal vitamin D deficiency exposure in later life.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
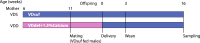




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

