Combination of US hyperthermia and radiotherapy on a preclinical glioblastoma model
- PMID: 39191985
- PMCID: PMC11350105
- DOI: 10.1038/s41598-024-70838-0
Combination of US hyperthermia and radiotherapy on a preclinical glioblastoma model
Abstract
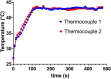
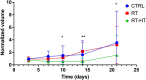
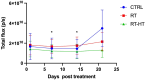
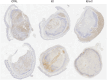
In this work the effect of combining ultrasound (US) hyperthermia (HT) with radiotherapy (RT) was investigated. The treatment was applied to a GBM xenograft nude mouse model obtained by injecting U87 luc+ cells. The combined treatment group received 6 Gy and HT at for 8 min. The ultrasound field was generated by a closed-loop computationally controlled system, consisting of a High Intensity Focused Ultrasound (HIFU) transducer with centre frequency 3.57 MHz, a power amplifier, a function generator and a MATLAB controller. A mechanical cone adaptor has been designed to use the HIFU beam at a pre-defined post-focal distance. Two thermocouples were placed between the mechanical cone and the mice skin to measure and control the temperature during the HT treatment. Radiotherapy was carried out by using a dedicated small animal image guided radiotherapy system. Measurements of tumor volume performed with a caliper showed good tumor control for the RT-HT group with respect to the RT or control groups for up to 21 days after treatment. The mean value of the normalized (before therapy) tumor volume was almost equal to 0.5 for two weeks after treatment with an increase to 1.5 at sacrifice. The control and HT groups showed a higher value of about 1.5 during the first two weeks and 3.5 at the end of the follow-up period. We concluded that the use of HT as a radiosensitizer can improve the outcome for glioblastoma treatments.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


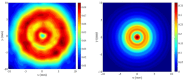
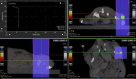
References
MeSH terms
Grants and funding
- 27336/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 18HLT06/European Association of National Metrology Institutes (EURAMET)
- 18HLT06/European Association of National Metrology Institutes (EURAMET)
- 18HLT06/European Association of National Metrology Institutes (EURAMET)
- 18HLT06/European Association of National Metrology Institutes (EURAMET)
LinkOut - more resources
Full Text Sources

