Electrical brain networks before and after transcranial pulsed shockwave stimulation in Alzheimer's patients
- PMID: 39192004
- PMCID: PMC11872817
- DOI: 10.1007/s11357-024-01305-x
Electrical brain networks before and after transcranial pulsed shockwave stimulation in Alzheimer's patients
Abstract
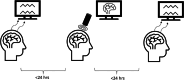
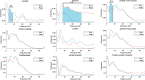
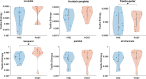
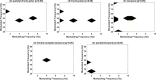
Alzheimer's disease (AD) is a neurodegenerative disorder that dramatically affects cognitive abilities and represents the most common cause of dementia. Currently, pharmacological interventions represent the main treatment to deal with the symptoms of AD; however, alternative approaches are readily sought. Transcranial pulse stimulation (TPS) is an emerging non-invasive neuromodulation technique that uses short, repetitive shockwaves with the potential to provide a wide range of vascular, metabolic, and neurotrophic changes and that has recently been shown to improve cognitive abilities in AD. This exploratory study aims to gain insight into the neurophysiological effect of one session of TPS in AD as reflected in electroencephalographic measures, e.g., spectral power, coherence, Tsallis entropy (TE), and cross-frequency coupling (cfc). We document changes in power (frontal and occipital), coherence (frontal, occipital and temporal), and TE (temporal and frontal) as well as changes in cfc (parietal-frontal, parietal-temporal, frontal-temporal). Our results emphasize the role of electroencephalographic measures as prospective markers for the neurophysiological effect of TPS.
Keywords: Alzheimer’s disease; Coherence; Cross-frequency-coupling; Electroencephalogram, ultrasound, shock waves; Non-pharmacological, intervention; Power; Tsallis entropy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This EEG study was conducted in accordance with the Ethics Committee of the regional Medical Chamber (Ärztekammer Nordrhein, Nr. 2021137). Patients signed a written consent for participation. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. Consent for publication: The authors have the right to publish the data expressed in the manuscript. Competing interests: LW has previously received funding grants and institutional support from the German Research Foundation, Hilde-Ulrichs-Stiftung für Parkinsonforschung, and the ParkinsonFonds Germany, BMBF/ERA-NETNEURON, DFG Forschergruppe (FOR1328), Deutsche Parkinson Vereinigung (DPV), Forschungskommission, Medizinische Fakultät, HHU Düsseldorf, UCB; Medtronic, UCB, Teva, Allergan, Merz, Abbvie, Roche, Bial, Merck, Novartis, Desitin, Spectrum. Lars Wojtecki owns stock in company BioNTech SE. Lars Wojtecki is a consultant to the following companies: TEVA, UCB Schwarz, Desitin, Medtronic, Abbott/Abbvie, MEDA, Boehringer I, Storz Medical, Kyowa Kirin, Guidepoint, Merck, Merz, Synergia, BIAL, Zambon, Sapio Life, STADA, Inomed, Vertanical. The other authors have no competing interests to declare.
Figures


References
-
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. 10.1056/NEJMoa2212948. - PubMed
-
- Mullard A. Landmark Alzheimer’s drug approval confounds research community. Nature. 2021;594:309–10. 10.1038/d41586-021-01546-2. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

