Heterochromatin formation and remodeling by IRTKS condensates counteract cellular senescence
- PMID: 39192031
- PMCID: PMC11480336
- DOI: 10.1038/s44318-024-00212-3
Heterochromatin formation and remodeling by IRTKS condensates counteract cellular senescence
Abstract
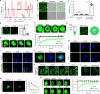
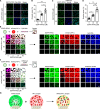
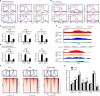
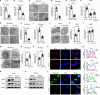
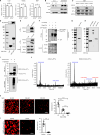
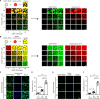
Heterochromatin, a key component of the eukaryotic nucleus, is fundamental to the regulation of genome stability, gene expression and cellular functions. However, the factors and mechanisms involved in heterochromatin formation and maintenance still remain largely unknown. Here, we show that insulin receptor tyrosine kinase substrate (IRTKS), an I-BAR domain protein, is indispensable for constitutive heterochromatin formation via liquid‒liquid phase separation (LLPS). In particular, IRTKS droplets can infiltrate heterochromatin condensates composed of HP1α and diverse DNA-bound nucleosomes. IRTKS can stabilize HP1α by recruiting the E2 ligase Ubc9 to SUMOylate HP1α, which enables it to form larger phase-separated droplets than unmodified HP1α. Furthermore, IRTKS deficiency leads to loss of heterochromatin, resulting in genome-wide changes in chromatin accessibility and aberrant transcription of repetitive DNA elements. This leads to activation of cGAS-STING pathway and type-I interferon (IFN-I) signaling, as well as to the induction of cellular senescence and senescence-associated secretory phenotype (SASP) responses. Collectively, our findings establish a mechanism by which IRTKS condensates consolidate constitutive heterochromatin, revealing an unexpected role of IRTKS as an epigenetic mediator of cellular senescence.
Keywords: Cellular Senescence; Heterochromatin Formation; IRTKS; Liquid-Liquid Phase Separation; SUMOylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
- 2022YFA1302700/MOST | National Key Research and Development Program of China (NKPs)
- 82073116/MOST | National Natural Science Foundation of China (NSFC)
- 82272969/MOST | National Natural Science Foundation of China (NSFC)
- 82203262/MOST | National Natural Science Foundation of China (NSFC)
- 21JC1403200/National Science Foundation (NSF)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

