Impact of a package of point-of-care diagnostic tests, a clinical diagnostic algorithm and adherence training on antibiotic prescriptions for the management of non-severe acute febrile illness in primary health facilities during the COVID-19 pandemic in Burkina Faso
- PMID: 39192209
- PMCID: PMC11351252
- DOI: 10.1186/s12879-024-09787-y
Impact of a package of point-of-care diagnostic tests, a clinical diagnostic algorithm and adherence training on antibiotic prescriptions for the management of non-severe acute febrile illness in primary health facilities during the COVID-19 pandemic in Burkina Faso
Abstract
Objective: To assess the impact of an intervention package on the prescription of antibiotic and subsequently the rate of clinical recovery for non-severe acute febrile illnesses at primary health centers.
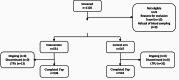
Methods: Patients over 6 months of age presenting to primary health care centres with fever or history of fever within the past 7 days were randomized to receive either the intervention package constituted of point-of-care tests including COVID-19 antigen tests, a diagnostic algorithm and training and communication packages, or the standard practice. The primary outcomes were antibiotic prescriptions at Day 0 (D0) and the clinical recovery at Day 7 (D7). Secondary outcomes were non-adherence of participants and parents/caregivers to prescriptions, health workers' non-adherence to the algorithm, and the safety of the intervention.
Results: A total of 1098 patients were enrolled. 551 (50.2%) were randomized to receive the intervention versus 547 (49.8%) received standard care. 1054 (96.0%) completed follow-up and all of them recovered at D7 in both arms. The proportion of patients with antibiotic prescriptions at D0 were 33.2% (183/551) in the intervention arm versus 58.1% (318/547) under standard care, risk difference (RD) -24.9 (95% CI -30.6 to -19.2, p < 0.001), corresponding to one more antibiotic saved every four (95% CI: 3 to 5) consultations. This reduction was also statistically significant in children from 6 to 59 months (RD -34.5; 95% CI -41.7 to -27.3; p < 0.001), patients over 18 years (RD -35.9; 95%CI -58.5 to -13.4; p = 0.002), patients with negative malaria test (RD -46.9; 95% CI -53.9 to -39.8; p < 0.001), those with a respiratory diagnosis (RD -48.9; 95% CI -56.9 to -41.0, p < 0.001) and those not vaccinated against COVID-19 (-24.8% 95%CI -30.7 to -18.9, p-value: <0.001). A significant reduction in non-adherence to prescription by patients was reported (RD -7.1; 95% CI -10.9 to -3.3; p < 0.001).
Conclusion: The intervention was associated with significant reductions of antibiotic prescriptions and non-adherence, chiefly among patients with non-malaria fever, those with respiratory symptoms and children below 5 years of age. The addition of COVID-19 testing did not have a major impact on antibiotic use at primary health centers.
Trial registration: Clinitrial.gov; NCT04081051 registered on 06/09/2019.
Keywords: Acute fever; Antibiotic prescription; COVID-19; Point-of-care tests; Respiratory tract infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization. WHO Informal Consultation on Fever Management in Peripheral Health Care settings: A Global Review of evidence and practice. WHO. 2013.
-
- O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Vol. 10, The Lancet Infectious Diseases. 2010. pp. 545–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous