MicroRNA mediated suppression of airway lactoperoxidase by TGF-β1 and cigarette smoke promotes airway inflammation
- PMID: 39192275
- PMCID: PMC11348649
- DOI: 10.1186/s12950-024-00405-x
MicroRNA mediated suppression of airway lactoperoxidase by TGF-β1 and cigarette smoke promotes airway inflammation
Abstract
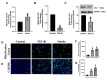
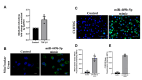
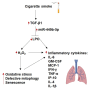
Transforming Growth Factor Beta1 (TGF-β1) signaling is upregulated in Chronic Obstructive Pulmonary disease (COPD), smokers, and people living with HIV. Cigarette smoking and HIV are also independent risk factors for COPD. Chronic inflammation is a hallmark of COPD. However, the underlying mechanisms remain unknown. Previous research has suggested that TGF-β1 alters the airway epithelial microRNAome and transcriptome, potentially contributing to lung inflammation. The Lactoperoxidase (LPO) system is an integral component of innate immunity within the airway. LPO plays a crucial role in host defense by catalyzing the oxidation of thiocyanate to hypothiocyanite in the presence of hydrogen peroxide (H2O2), generating a potent antibacterial and antiviral agent. Additionally, the LPO system potentially aids in maintaining cellular redox balance by reducing the levels of H2O2, thus mitigating oxidative stress within the airway epithelium. LPO dysfunction can impair immune responses and exacerbate inflammatory processes in respiratory diseases.In this study, primary bronchial epithelial cells and bronchial cell lines were treated with TGF-β1 and exposed to cigarette smoke to characterize the effect of these factors on LPO and their downstream effects. RT-qPCR and Western Blot were applied to quantify mRNA and proteins' expression. The levels of H2O2 were detected using the Amplex Red Assay. Magnetofection and transfection were applied to probe the effect of miR-449b-5p. Staining procedures using the MitoTracker Green and C12FDG dyes were used to establish mitochondria mass and senescence. The levels of pro-inflammatory cytokines were measured via Luminex assays.We found that TGF-β1 and cigarette smoke suppressed airway LPO expression, increasing H2O2 levels. This increase in H2O2 had downstream effects on mitochondrial homeostasis, epithelial cellular senescence, and the pro-inflammatory cytokine response. We demonstrate for the first time that airway LPO is regulated by TGF-β1-induced miRNA-mediated post-transcriptional silencing through miR-449b-5p in the lungs. Further, we identify and validate miR-449-5p as the candidate miRNA upregulated by TGF-β1, which is involved in LPO suppression. This paper demonstrates a new mechanism by which TGF-β1 can lead to altered redox status in the airway.
Keywords: COPD; Cigarette smoke; IL-6; Inflammation; LPO; TGF-β1; miR-449b-5p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

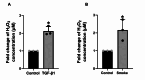
References
-
- Larsen DL, Gandhi H, Pollack M, Feigler N, Patel S, Wise RA. The quality of care and economic burden of COPD in the United States: considerations for managing patients and improving outcomes. Am Health Drug Benefits. 2022;15(2):57–64.
Grants and funding
LinkOut - more resources
Full Text Sources

