MiR-454-3p regulates high glucose-induced mesothelial-mesenchymal transition and glycolysis in peritoneal mesothelial cells by targeting STAT3
- PMID: 39192609
- PMCID: PMC11360635
- DOI: 10.1080/0886022X.2024.2394635
MiR-454-3p regulates high glucose-induced mesothelial-mesenchymal transition and glycolysis in peritoneal mesothelial cells by targeting STAT3
Abstract
Background: The quality of life of patients receiving long-term peritoneal dialysis (PD) is significantly impacted by the onset of peritoneal fibrosis (PF), and one of the pathological changes is mesothelial-mesenchymal transition (MMT). In this study, we investigated the potential roles of miR-454-3p and signal transducer and activator of transcription 3 (STAT3) in the progression of peritoneal MMT and the underlying mechanisms.
Methods: Peritoneums were collected to detect morphology via hematoxylin-eosin staining and differentially expressed miRNAs were detected via RT-qPCR. PD effluent-derived cell populations in the peritoneal cavity were isolated from the effluents of 20 PD patients to determine miR-454-3p, STAT3, and MMT markers via Western blotting and RT-qPCR. The relationship between miR-454-3p and STAT3 was examined via a dual-luciferase reporter assay. Western blotting and RT-qPCR were utilized to evaluate the expression of STAT3, MMT markers, and glycolytic enzymes. Immunofluorescence staining revealed the localization and expression of MMT markers and STAT3.
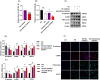
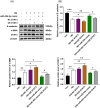
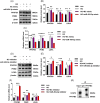
Results: MiR-454-3p was downregulated in the peritoneums and PD effluent-derived cell populations of long-term PD patients. High glucose (HG) treatment promoted HMrSV5 cell MMT and glycolysis. MiR-454-3p overexpression alleviated HG-induced MMT and suppressed the expression of STAT3 and glycolytic enzymes. In contrast, the miR-454-3p inhibitor exacerbated HG-induced MMT and promoted the expression of glycolytic enzymes and STAT3. Moreover, STAT3 was the target of miR-454-3p.
Conclusions: This study demonstrated the protective role of miR-454-3p in HG-induced MMT and glycolysis in HMrSv5 cells, suggesting that miR-454-3p may prevent MMT by suppressing glycolytic enzymes via the STAT3/PFKFB3 pathway in the HG environment.
Keywords: Glycolysis; STAT3; mesothelial-mesenchymal transition (MMT); miR-454-3p; peritoneal fibrosis.
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
