Innovation and optimization in autoimmune encephalitis trials: the design and rationale for the Phase 3, randomized study of satralizumab in patients with NMDAR-IgG-antibody-positive or LGI1-IgG-antibody-positive autoimmune encephalitis (CIELO)
- PMID: 39193150
- PMCID: PMC11348855
- DOI: 10.3389/fneur.2024.1437913
Innovation and optimization in autoimmune encephalitis trials: the design and rationale for the Phase 3, randomized study of satralizumab in patients with NMDAR-IgG-antibody-positive or LGI1-IgG-antibody-positive autoimmune encephalitis (CIELO)
Abstract
Background: Autoimmune encephalitis (AIE) encompasses a spectrum of rare autoimmune-mediated neurological disorders, which are characterized by brain inflammation and dysfunction. Autoantibodies targeting the N-methyl-d-aspartic acid receptor (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1) are the most common subtypes of antibody-positive AIE. Currently, there are no approved therapies for AIE. Interleukin-6 (IL-6) signaling plays a role in the pathophysiology of AIE. Satralizumab, a humanized, monoclonal recycling antibody that specifically targets the IL-6 receptor and inhibits IL-6 signaling, has demonstrated efficacy and safety in another autoantibody-mediated neuroinflammatory disease, aquaporin-4 immunoglobulin G antibody-positive neuromyelitis optica spectrum disorder, and has the potential to be an evidence-based disease modifying treatment in AIE.
Objectives: CIELO will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of satralizumab compared with placebo in patients with NMDAR-immunoglobulin G antibody-positive (IgG+) or LGI1-IgG+ AIE.
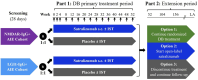
Study design: CIELO (NCT05503264) is a prospective, Phase 3, randomized, double-blind, multicenter, basket study that will enroll approximately 152 participants with NMDAR-IgG+ or LGI1-IgG+ AIE. Prior to enrollment, participants will have received acute first-line therapy. Part 1 of the study will consist of a 52-week primary treatment period, where participants will receive subcutaneous placebo or satralizumab at Weeks 0, 2, 4, and every 4 weeks thereafter. Participants may continue to receive background immunosuppressive therapy, symptomatic treatment, and rescue therapy throughout the study. Following Part 1, participants can enter an optional extension period (Part 2) to continue the randomized, double-blind study drug, start open-label satralizumab, or stop study treatment and continue with follow-up assessments.
Endpoints: The primary efficacy endpoint is the proportion of participants with a ≥1-point improvement in the modified Rankin Scale (mRS) score from study baseline and no use of rescue therapy at Week 24. Secondary efficacy assessments include mRS, Clinical Assessment Scale of Autoimmune Encephalitis (CASE), time to rescue therapy, sustained seizure cessation and no rescue therapy, Montreal Cognitive Assessment, and Rey Auditory Verbal Learning Test (RAVLT) measures. Safety, pharmacokinetics, pharmacodynamics, exploratory efficacy, and biomarker endpoints will be captured.
Conclusion: The innovative basket study design of CIELO offers the opportunity to yield prospective, robust evidence, which may contribute to the development of evidence-based treatment recommendations for satralizumab in AIE.
Keywords: N-methyl-d-aspartic acid receptor (NMDAR); autoimmune encephalitis (AIE); clinical trial; leucine-rich glioma-inactivated 1 (LGI1); satralizumab.
Copyright © 2024 Lee, Abboud, Irani, Nakajima, Piquet, Pittock, Yeh, Wang, Rajan, Overell, Smith, St Lambert, El-Khairi, Gafarova and Gelfand.
Conflict of interest statement
S-TL reports advisory roles for Roche/Genentech, Biofire Diagnostics, GC Pharma, Celltrion, and Advanced Neural Technologies. HA is a consultant and speaker for Biogen, Genentech, BMS, Horizon, and Alexion. HA has served as a consultant and/or advisory board member for Cycle Pharma, Alpine Pharma, and Axonics. HA received honoraria from Neurology Live. HA receives research support from Genentech, BMS, Novartis, Sanofi, UCB, and the Guthy-Jackson Charitable Foundation. HA receives royalties from UpToDate. HA serves as an Assistant Editor for Neurology Journal. SI has received honoraria/research support from UCB, Immunovant, Roche, Janssen, Cerebral Therapeutics, ADC Therapeutics, Brain, CSL Behring, and ONO Pharma. SI receives licensed royalties on patent application WO/2010/046716 entitled ‘Neurological Autoimmune Disorders,’ and has filed two other patents entitled “Diagnostic method and therapy” (WO2019211633 and US-2021-0071249-A1; PCT application WO202189788A1) and “Biomarkers” (PCT/GB2022/050614 and WO202189788A1). HN reports being a consultant/advisor for F. Hoffmann-La Roche Ltd. and has received speaker honoraria from Alexion, Biogen, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Kirin, and Takeda. AP reports research grants from the University of Colorado, Rocky Mountain MS Center, and the Foundation for Sarcoidosis; consulting fees from Genentech/Roche, UCB, EMD Serono, and Alexion; and honoraria from MedLink and publication royalties from Springer as co-editor of a medical textbook. AP serves as an unpaid member of the Medical Advisory Board for the Autoimmune Encephalitis Alliance. SP reports grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals; grants, personal fees, and nonfinancial support from MedImmune, Viela Bio, and Horizon; grants from Adimune; grants, personal fees, and nonfinancial support from Genentech and Roche, and personal fees for consulting from UCB, Astellas, and Arialys. SP has two patents issued (8889102; application 12-678350; Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia; and 9891219B2; application 12-573942; Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an Individual That is Aquaporin-4 [AQP4]-IgG Autoantibody-Positive), for which he has received royalties. SP also has patents pending for IgGs to the following proteins as biomarkers of autoimmune neurological disorders: septin-5, kelch-like protein 11, GFAP, PDE10A, and MAP1B. SP is an employee of Mayo Clinic, which offers commercial NMDAR and LGI1-IgG testing. SP receives no royalties from the sale of tests done at the neuroimmunology laboratory at Mayo Clinic. EY has received research funding from NMSS, CMSC, CIHR, NIH, OIRM, SCN, CBMH Chase an Idea, SickKids Foundation, Rare Diseases Foundation, MS Scientific Foundation, McLaughlin Centre, Leong Center, and Peterson Foundation, and investigator-initiated research funding from Biogen. EY has served on a scientific advisory board for F. Hoffmann-La Roche and a data safety monitoring board for TG Therapeutics and has received speaker honoraria from Biogen, JHU, Saudi Epilepsy Society, NYU, MS-ATL, ACRS, PRIME, and CNPS. JW serves as a trial steering committee member for the CIELO study. JO and MG are employees and shareholders of F. Hoffmann-La Roche Ltd. JS, JSL, and ME-K are employees of Roche Products Ltd. and shareholders of F. Hoffmann-La Roche Ltd. SR is an employee and shareholder of Genentech, Inc. JG receives research support to his institution from F. Hoffmann-La Roche Ltd. for clinical trials and serves as chair of the Trial Steering Committee for the CIELO study. JG has also received research support to his institution for clinical trials from Vigil Neurosciences; and received personal fees for consulting for Arialys and Ventyx Bio. The authors declare that this study received funding from F. Hoffmann-La Roche. The sponsor had the following involvement in the study: study design, collection, analysis and the decision to submit it for publication. Medical writing assistance was provided by Reece Bracewell, MBiolSci and Nicole Ellman, PhD, of Apothecom, London, UK, and was funded by F. Hoffmann-La Roche Ltd. All authors contributed to the conceptualisation, drafting, revising, and critical reviewing of the manuscript for intellectual content, and approved the final version of this manuscript to be published.
Figures
References
-
- Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. (2013) 57:1114–28. doi: 10.1093/cid/cit458, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

