The gut-lung axis: the impact of the gut mycobiome on pulmonary diseases and infections
- PMID: 39193472
- PMCID: PMC11316619
- DOI: 10.1093/oxfimm/iqae008
The gut-lung axis: the impact of the gut mycobiome on pulmonary diseases and infections
Abstract
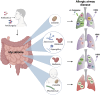
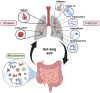
The gastrointestinal tract contains a diverse microbiome consisting of bacteria, fungi, viruses and archaea. Although these microbes usually reside as commensal organisms, it is now well established that higher abundance of specific bacterial or fungal species, or loss of diversity in the microbiome can significantly affect development, progression and outcomes in disease. Studies have mainly focused on the effects of bacteria, however, the impact of other microbes, such as fungi, has received increased attention in the last few years. Fungi only represent around 0.1% of the total gut microbial population. However, key fungal taxa such as Candida, Aspergillus and Wallemia have been shown to significantly impact health and disease. The composition of the gut mycobiome has been shown to affect immunity at distal sites, such as the heart, lung, brain, pancreas, and liver. In the case of the lung this phenomenon is referred to as the 'gut-lung axis'. Recent studies have begun to explore and unveil the relationship between gut fungi and lung immunity in diseases such as asthma and lung cancer, and lung infections caused by viruses, bacteria and fungi. In this review we will summarize the current, rapidly growing, literature describing the impact of the gut mycobiome on respiratory disease and infection.
Keywords: Mycobiome; gut-lung axis; lung infections.
© The Author(s) 2024. Published by Oxford University Press.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
