Heterochromatin protein 1 alpha (HP1α) undergoes a monomer to dimer transition that opens and compacts live cell genome architecture
- PMID: 39193905
- PMCID: PMC11472067
- DOI: 10.1093/nar/gkae720
Heterochromatin protein 1 alpha (HP1α) undergoes a monomer to dimer transition that opens and compacts live cell genome architecture
Abstract
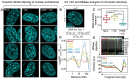
Our understanding of heterochromatin nanostructure and its capacity to mediate gene silencing in a living cell has been prevented by the diffraction limit of optical microscopy. Thus, here to overcome this technical hurdle, and directly measure the nucleosome arrangement that underpins this dense chromatin state, we coupled fluorescence lifetime imaging microscopy (FLIM) of Förster resonance energy transfer (FRET) between histones core to the nucleosome, with molecular editing of heterochromatin protein 1 alpha (HP1α). Intriguingly, this super-resolved readout of nanoscale chromatin structure, alongside fluorescence fluctuation spectroscopy (FFS) and FLIM-FRET analysis of HP1α protein-protein interaction, revealed nucleosome arrangement to be differentially regulated by HP1α oligomeric state. Specifically, we found HP1α monomers to impart a previously undescribed global nucleosome spacing throughout genome architecture that is mediated by trimethylation on lysine 9 of histone H3 (H3K9me3) and locally reduced upon HP1α dimerisation. Collectively, these results demonstrate HP1α to impart a dual action on chromatin that increases the dynamic range of nucleosome proximity. We anticipate that this finding will have important implications for our understanding of how live cell heterochromatin structure regulates genome function.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Klemm S.L., Shipony Z., Greenleaf W.J.. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019; 20:207–220. - PubMed
-
- Maison C., Almouzni G.. HP1 and the dynamics of heterochromatin maintenance. Nat. Rev. Mol. Cell Biol. 2004; 5:296–304. - PubMed
-
- Jacobs S.A., Khorasanizadeh S.. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002; 295:2080–2083. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

