Phosphorylation of the selective autophagy receptor TAX1BP1 by TBK1 and IKBKE/IKKi promotes ATG8-family protein-dependent clearance of MAVS aggregates
- PMID: 39193925
- PMCID: PMC11702929
- DOI: 10.1080/15548627.2024.2394306
Phosphorylation of the selective autophagy receptor TAX1BP1 by TBK1 and IKBKE/IKKi promotes ATG8-family protein-dependent clearance of MAVS aggregates
Abstract
TAX1BP1 is a selective macroautophagy/autophagy receptor that inhibits NFKB and RIGI-like receptor (RLR) signaling to prevent excessive inflammation and maintain homeostasis. Selective autophagy receptors such as SQSTM1/p62 and OPTN are phosphorylated by the kinase TBK1 to stimulate their selective autophagy function. However, it is unknown if TAX1BP1 is regulated by TBK1 or other kinases under basal conditions or during RNA virus infection. Here, we found that TBK1 and IKBKE/IKKi function redundantly to phosphorylate TAX1BP1 and regulate its autophagic turnover through canonical macroautophagy. TAX1BP1 phosphorylation promotes its localization to lysosomes, resulting in its degradation. Additionally, we found that during vesicular stomatitis virus infection, TAX1BP1 is targeted to lysosomes in an ATG8-family protein-independent manner. Furthermore, TAX1BP1 plays a critical role in the clearance of MAVS aggregates, and phosphorylation of TAX1BP1 controls its MAVS aggrephagy function. Together, our data support a model whereby TBK1 and IKBKE license TAX1BP1-selective autophagy function to inhibit MAVS and RLR signaling.Abbreviations: ATG: autophagy related; BafA1: bafilomycin A1; CALCOCO2: calcium binding and coiled-coil domain 2; GFP: green fluorescent protein; IFA: indirect immunofluorescence assay; IFN: interferon; IκB: inhibitor of nuclear factor kappa B; IKK: IκB kinase; IRF: interferon regulatory factor; KO: knockout; LAMP1: lysosomal associated membrane protein 1; LIR: LC3-interacting region; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MAVS: mitochondrial antiviral signaling protein; MEF: mouse embryonic fibroblast; MOI: multiplicity of infection; IKBKG/NEMO: inhibitor of nuclear factor kappa B kinase regulatory subunit gamma; NFKB: nuclear factor kappa B; OPTN: optineurin; Poly(I:C): polyinosinic-polycytidylic acid; RB1CC1/FIP200: RB1 inducible coiled-coil 1; RIGI: RNA sensor RIG-I; RLR: RIGI-like receptor; SDD-AGE: semi-denaturing detergent-agarose gel electrophoresis; SeV: Sendai virus; SLR: SQSTM1-like receptor; SQSTM1: sequestosome 1; TAX1BP1: Tax1 binding protein 1; TBK1: TANK binding kinase 1; TNF: tumor necrosis factor; TRAF: TNF receptor associated factor; VSV: vesicular stomatitis virus; ZnF: zinc finger.
Keywords: Aggrephagy; IKBKE/IKKi; MAVS; TAX1BP1; TBK1; autophagy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



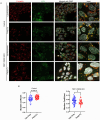



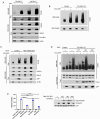
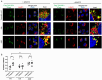
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
