MYC Induces Oncogenic Stress through RNA Decay and Ribonucleotide Catabolism in Breast Cancer
- PMID: 39193992
- PMCID: PMC11372365
- DOI: 10.1158/2159-8290.CD-22-0649
MYC Induces Oncogenic Stress through RNA Decay and Ribonucleotide Catabolism in Breast Cancer
Abstract
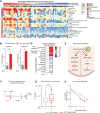
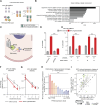
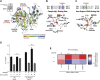
Upregulation of MYC is a hallmark of cancer, wherein MYC drives oncogenic gene expression and elevates total RNA synthesis across cancer cell transcriptomes. Although this transcriptional anabolism fuels cancer growth and survival, the consequences and metabolic stresses induced by excess cellular RNA are poorly understood. Herein, we discover that RNA degradation and downstream ribonucleotide catabolism is a novel mechanism of MYC-induced cancer cell death. Combining genetics and metabolomics, we find that MYC increases RNA decay through the cytoplasmic exosome, resulting in the accumulation of cytotoxic RNA catabolites and reactive oxygen species. Notably, tumor-derived exosome mutations abrogate MYC-induced cell death, suggesting excess RNA decay may be toxic to human cancers. In agreement, purine salvage acts as a compensatory pathway that mitigates MYC-induced ribonucleotide catabolism, and inhibitors of purine salvage impair MYC+ tumor progression. Together, these data suggest that MYC-induced RNA decay is an oncogenic stress that can be exploited therapeutically. Significance: MYC is the most common oncogenic driver of poor-prognosis cancers but has been recalcitrant to therapeutic inhibition. We discovered a new vulnerability in MYC+ cancer where MYC induces cell death through excess RNA decay. Therapeutics that exacerbate downstream ribonucleotide catabolism provide a therapeutically tractable approach to TNBC (Triple-negative Breast Cancer) and other MYC-driven cancers.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.K. Meena reports grants from Susan G. Komen during the conduct of the study; personal fees from Affirmativ Diagnostics DBA ADx Health outside the submitted work. E.A. Bowling reports grants from NIH/NCI during the conduct of the study. K. Eagle reports personal fees from Flare Therapeutics and personal fees from Dana Farber Cancer Institute outside the submitted work. A.D. Yang reports grants from CPRIT and NIH/NCI during the conduct of the study. S.J. Kurley reports grants from CDMRP and The Helis Foundation during the conduct of the study; personal fees from Optum Genomics (UnitedHealth Group) and personal fees from Castle Biosciences outside the submitted work. C.M. Olson reports personal fees from Marlinspike Therapeutics outside the submitted work. K.L. Meerbrey reports personal fees from Marlinspike Therapeutics outside the submitted work. A. Sreekumar reports grants from Agilent Foundation, non-financial support from Sri Sathya Sai Institute for Higher Learning, India, and personal fees from Karkinos Health Care Pvt. Ltd., India, outside the submitted work. D. Rejman reports grants from The Ministry of Education, Youth and Sports during the conduct of the study. C.Y. Lin reports other support from Kronos Bio outside the submitted work. M.C. Pillon reports grants from Cancer Prevention & Research Institute of Texas, National Institute of Environmental Health Sciences, and National Institute of General Medical Sciences during the conduct of the study. T.F. Westbrook reports grants, personal fees, and other support from Marlinspike Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures


References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. - PubMed
-
- Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. Recent Results Cancer Res 2016;207:39–72. - PubMed
-
- Sullivan LB, Gui DY, Van Der Heiden MG. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 2016;16:680–93. - PubMed
MeSH terms
Substances
Grants and funding
- R35GM147123/National Institute of General Medical Sciences (NIGMS)
- T32 GM120011/GM/NIGMS NIH HHS/United States
- 1F30CA278316-01/National Cancer Institute (NCI)
- RR200076/Cancer Prevention and Research Institute of Texas (CPRIT)
- R00 ES030735/ES/NIEHS NIH HHS/United States
- 1R01CA215452-01/National Cancer Institute (NCI)
- P30 CA125123/CA/NCI NIH HHS/United States
- Q-0007/Welch Foundation (The Welch Foundation)
- T32GM120011/National Institutes of Health (NIH)
- RR150093/Cancer Prevention and Research Institute of Texas (CPRIT)
- R01 CA215226/CA/NCI NIH HHS/United States
- C5470/A27144/Mark Foundation For Cancer Research (The Mark Foundation for Cancer Research)
- R01 CA215452/CA/NCI NIH HHS/United States
- U01CA214125/National Cancer Institute (NCI)
- PDF17487931/Susan G. Komen (SGK)
- R00ES030735/National Institute of Environmental Health Sciences (NIEHS)
- 1W81XWH-18-1-0573/U.S. Department of Defense (DOD)
- LX22NPO5103/National Institute of Virology and Bacteriology
- 1R01CA215226/National Cancer Institute (NCI)
- P30 DK056338/DK/NIDDK NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- U01 CA214125/CA/NCI NIH HHS/United States
- F30 CA278316/CA/NCI NIH HHS/United States
- R35 GM147123/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

