Pharmacokinetics and safety of TBAJ-876, a novel antimycobacterial diarylquinoline, in healthy subjects
- PMID: 39194204
- PMCID: PMC11460996
- DOI: 10.1128/aac.00613-24
Pharmacokinetics and safety of TBAJ-876, a novel antimycobacterial diarylquinoline, in healthy subjects
Abstract
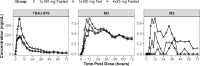
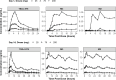
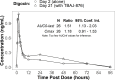
TBAJ-876, a second-generation diarylquinoline with greater antimycobacterial activity and a potentially better safety profile compared with bedaquiline, is under development for the treatment of drug-susceptible and drug-resistant tuberculosis (TB). A phase 1, first-in-human study of TBAJ-876, comprising a single-ascending dose (SAD) part including a food effect cohort, a multiple-ascending dose (MAD) part, and a relative bioavailability part of tablets versus oral suspension, was conducted on 137 healthy adults. A drug-drug interaction study was conducted on 28 healthy adults to evaluate the effects of TBAJ-876 on a cytochrome P450 3A4 substrate (midazolam) and a P-glycoprotein substrate (digoxin). TBAJ-876 was well-tolerated at single doses up to 800 mg and multiple doses up to 200 mg for 14 days. No deaths or serious adverse events occurred. No episodes of clinically significant prolongation of the QTc interval were observed. TBAJ-876 exposures were dose proportional in the SAD and MAD studies. TBAJ-876 exhibited multicompartmental pharmacokinetics (PK) with a long terminal half-life yielding quantifiable concentrations up to the longest follow-up of 10 weeks after a single dose and resulting in accumulation with multiple dosing. In the fed state, TBAJ-876 exposures approximately doubled with the tablet formulation, whereas M3 metabolite exposures decreased by approximately 20%. The relative bioavailability of TBAJ-876 was similar between tablets and the oral suspension at 100-mg doses. With co-administration of TBAJ-876, the AUC0-inf of midazolam was unchanged and the Cmax was reduced by 14%; the AUC0-last of digoxin was increased by 51%, and the Cmax was increased by 18%. These results support further investigation of TBAJ-876 for the treatment of tuberculosis.
Keywords: TBAJ-876; antimicrobial safety; diarylquinoline; pharmacokinetics; phase 1; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Global tuberculosis report. 2023. World Health Organization; Geneva
-
- WHO consolidated guidelines on tuberculosis. 2022. Geneva: World Health Organiztion
-
- Timm J, Bateson A, Solanki P, Paleckyte A, Witney AA, Rofael SAD, Fabiane S, Olugbosi M, McHugh TD, Sun E. 2023. Baseline and acquired resistance to bedaquiline, linezolid and pretomanid, and impact on treatment outcomes in four tuberculosis clinical trials containing pretomanid. PLOS Glob Public Health 3:e0002283. doi:10.1371/journal.pgph.0002283 - DOI - PMC - PubMed
-
- Satapathy P, Itumalla R, Neyazi A, Nabizai Taraki AM, Khatib MN, Gaidhane S, Zahiruddin QS, Rustagi S, Neyazi M. 2024. Emerging bedaquiline resistance: a threat to the global fight against drug-resistant tuberculosis. J Biosaf Biosec 6:13–15. doi:10.1016/j.jobb.2024.01.001 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

