Application of machine-learning models to predict the ganciclovir and valganciclovir exposure in children using a limited sampling strategy
- PMID: 39194260
- PMCID: PMC11459947
- DOI: 10.1128/aac.00860-24
Application of machine-learning models to predict the ganciclovir and valganciclovir exposure in children using a limited sampling strategy
Abstract
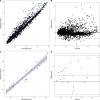
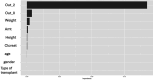
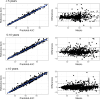
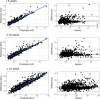
Intravenous ganciclovir and oral valganciclovir display significant variability in ganciclovir pharmacokinetics, particularly in children. Therapeutic drug monitoring currently relies on the area under the concentration-time (AUC). Machine-learning (ML) algorithms represent an interesting alternative to Maximum-a-Posteriori Bayesian-estimators for AUC estimation. The goal of our study was to develop and validate an ML-based limited sampling strategy (LSS) approach to determine ganciclovir AUC0-24 after administration of either intravenous ganciclovir or oral valganciclovir in children. Pharmacokinetic parameters from four published population pharmacokinetic models, in addition to the World Health Organization growth curve for children, were used in the mrgsolve R package to simulate 10,800 pharmacokinetic profiles of children. Different ML algorithms were trained to predict AUC0-24 based on different combinations of two or three samples. Performances were evaluated in a simulated test set and in an external data set of real patients. The best estimation performances in the test set were obtained with the Xgboost algorithm using a 2 and 6 hours post dose LSS for oral valganciclovir (relative mean prediction error [rMPE] = 0.4% and relative root mean square error [rRMSE] = 5.7%) and 0 and 2 hours post dose LSS for intravenous ganciclovir (rMPE = 0.9% and rRMSE = 12.4%). In the external data set, the performance based on these two sample LSS was acceptable: rMPE = 0.2% and rRMSE = 16.5% for valganciclovir and rMPE = -9.7% and rRMSE = 17.2% for intravenous ganciclovir. The Xgboost algorithm developed resulted in a clinically relevant individual estimation using only two blood samples. This will improve the implementation of AUC-targeted ganciclovir therapeutic drug monitoring in children.
Keywords: Xgboost; artificial intelligence; children; ganciclovir; machine learning; pharmacokinetics; pharmacometrics; transplantation; valganciclovir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Manuel O, Kralidis G, Mueller NJ, Hirsch HH, Garzoni C, van Delden C, Berger C, Boggian K, Cusini A, Koller MT, Weisser M, Pascual M, Meylan PR, Swiss Transplant Cohort Study . 2013. Impact of antiviral preventive strategies on the incidence and outcomes of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 13:2402–2410. doi:10.1111/ajt.12388 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

