Plasticity Comparison of Two Stem Cell Sources with Different Hox Gene Expression Profiles in Response to Cobalt Chloride Treatment during Chondrogenic Differentiation
- PMID: 39194498
- PMCID: PMC11352031
- DOI: 10.3390/biology13080560
Plasticity Comparison of Two Stem Cell Sources with Different Hox Gene Expression Profiles in Response to Cobalt Chloride Treatment during Chondrogenic Differentiation
Abstract
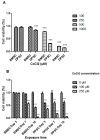
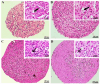
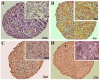
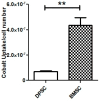
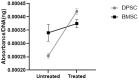
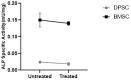
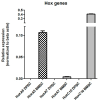
The limited self-repair capacity of articular cartilage is a challenge for healing injuries. While mesenchymal stem/stromal cells (MSCs) are a promising approach for tissue regeneration, the criteria for selecting a suitable cell source remain undefined. To propose a molecular criterion, dental pulp stem cells (DPSCs) with a Hox-negative expression pattern and bone marrow mesenchymal stromal cells (BMSCs), which actively express Hox genes, were differentiated towards chondrocytes in 3D pellets, employing a two-step protocol. The MSCs' response to preconditioning by cobalt chloride (CoCl2), a hypoxia-mimicking agent, was explored in an assessment of the chondrogenic differentiation's efficiency using morphological, histochemical, immunohistochemical, and biochemical experiments. The preconditioned DPSC pellets exhibited significantly elevated levels of collagen II and glycosaminoglycans (GAGs) and reduced levels of the hypertrophic marker collagen X. No significant effect on GAGs production was observed in the preconditioned BMSC pellets, but collagen II and collagen X levels were elevated. While preconditioning did not modify the ALP specific activity in either cell type, it was notably lower in the DPSCs differentiated pellets compared to their BMSCs counterparts. These results could be interpreted as demonstrating the higher plasticity of DPSCs compared to BMSCs, suggesting the contribution of their unique molecular characteristics, including their negative Hox expression pattern, to promote a chondrogenic differentiation potential. Consequently, DPSCs could be considered compelling candidates for future cartilage cell therapy.
Keywords: Hox genes; bone marrow mesenchymal stromal cell; chondrogenic differentiation; cobalt chloride; dental pulp stem cell; stem cell plasticity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Asadi-Golshan R., Razban V., Mirzaei E., Rahmanian A., Khajeh S., Mostafavi-Pour Z., Dehghani F. Efficacy of dental pulp-derived stem cells conditioned medium loaded in collagen hydrogel in spinal cord injury in rats: Stereological evidence. J. Chem. Neuroanat. 2021;116:101978. doi: 10.1016/j.jchemneu.2021.101978. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

