Antiarthritic and Antinociceptive Properties of Ylang-Ylang (Cananga odorata) Essential Oil in Experimental Models
- PMID: 39194751
- PMCID: PMC11352243
- DOI: 10.3390/cimb46080534
Antiarthritic and Antinociceptive Properties of Ylang-Ylang (Cananga odorata) Essential Oil in Experimental Models
Abstract
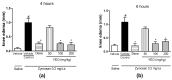
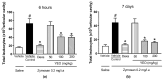
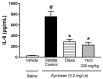
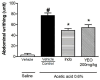
The aim of this study was to evaluate the effect of ylang-ylang (Cananga odorata) essential oil (YEO) on models of experimental arthritis, persistent inflammation, and nociception in mice. YEO treatment at doses of 100 and 200 mg/kg reduced the infiltration of leukocytes into the joint cavities of mice submitted to zymosan-induced arthritis 6 h and 7 days after arthritis induction. At these doses, YEO treatment reduced the formation of joint edema 4 and 6 h after arthritis induction, and at a dose of 200 mg/kg, YEO treatment reduced mechanical hyperalgesia 3 and 4 h after arthritis induction. At the dose of 200 mg/kg, YEO treatment reduced interleukin-6 (IL-6) levels and cartilage destruction in the zymosan-induced arthritis model, and reduced edema formation and mechanical hyperalgesia in the model of persistent inflammation (21 days) induced by complete Freund's adjuvant (CFA) in mice. YEO treatment at a dose of 200 mg/kg reduced the nociceptive response in experimental models of nociception induced by acetic acid and formalin. The YEO treatment reduced inflammatory parameters in the experimental arthritis model, and presented antiarthritic, anti-hyperalgesic, antinociceptive, and anti-inflammatory properties.
Keywords: arthritis; essential oil; natural products; persistent inflammation; ylang-ylang.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Souto F.O., Zarpelon A.C., Staurengo-Ferrari L., Fattori V., Casagrande R., Fonseca M.J.V., Cunha T.M., Ferreira S.H., Cunha F.Q., Verri W.A. Quercetin Reduces Neutrophil Recruitment Induced by CXCL8, LTB4, and FMLP: Inhibition of Actin Polymerization. J. Nat. Prod. 2011;74:113–118. doi: 10.1021/np1003017. - DOI - PubMed
LinkOut - more resources
Full Text Sources

