Sulfotransferase 1C2 Increases Mitochondrial Respiration by Converting Mitochondrial Membrane Cholesterol to Cholesterol Sulfate
- PMID: 39194960
- PMCID: PMC11411706
- DOI: 10.1021/acs.biochem.3c00344
Sulfotransferase 1C2 Increases Mitochondrial Respiration by Converting Mitochondrial Membrane Cholesterol to Cholesterol Sulfate
Abstract
Hypothesis: In this communication, we test the hypothesis that sulfotransferase 1C2 (SULT1C2, UniProt accession no. Q9WUW8) can modulate mitochondrial respiration by increasing state-III respiration.
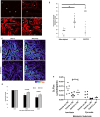
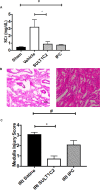
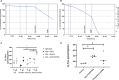
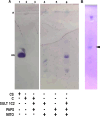
Methods and results: Using freshly isolated mitochondria, the addition of SULT1C2 and 3-phosphoadenosine 5 phosphosulfate (PAPS) results in an increased maximal respiratory capacity in response to the addition of succinate, ADP, and rotenone. Lipidomics and thin-layer chromatography of mitochondria treated with SULT1C2 and PAPS showed an increase in the level of cholesterol sulfate. Notably, adding cholesterol sulfate at nanomolar concentration to freshly isolated mitochondria also increases maximal respiratory capacity. In vivo studies utilizing gene delivery of SULT1C2 expression plasmids to kidneys result in increased mitochondrial membrane potential and confer resistance to ischemia/reperfusion injury. Mitochondria isolated from gene-transduced kidneys have elevated state-III respiration as compared with controls, thereby recapitulating results obtained with mitochondrial fractions treated with SULT1C2 and PAPS.
Conclusion: SULT1C2 increases mitochondrial respiratory capacity by modifying cholesterol, resulting in increased membrane potential and maximal respiratory capacity. This finding uncovers a unique role of SULT1C2 in cellular physiology and extends the role of sulfotransferases in modulating cellular metabolism.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Athanasiadis D.; Kapelouzou A.; Martikos G.; Katsimpoulas M.; Schizas D.; Vasdekis S. N.; Kostakis A.; Liakakos T. D.; Lazaris A. M. Remote Ischemic Preconditioning May Attenuate Renal Ischemia-Reperfusion Injury in a Porcine Model of Supraceliac Aortic Cross-Clamping. J. Vasc. Res. 2015, 52, 161–171. 10.1159/000439219. - DOI - PubMed
-
- Hussein A. M.; Sakr H. F.; Alenzi F. Q. Possible Underlying Mechanisms of the Renoprotective Effect of Remote Limb Ischemic Preconditioning Against Renal Ischemia/Reperfusion Injury: A Role of Osteopontin, Transforming Growth Factor-Beta and Survivin. Nephron 2016, 134, 117–129. 10.1159/000447953. - DOI - PubMed

