Analysis of Structural Changes of pH-Thermo-Responsive Nanoparticles in Polymeric Hydrogels
- PMID: 39195070
- PMCID: PMC11353694
- DOI: 10.3390/gels10080541
Analysis of Structural Changes of pH-Thermo-Responsive Nanoparticles in Polymeric Hydrogels
Abstract
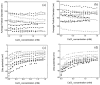
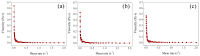
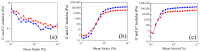
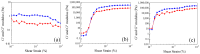
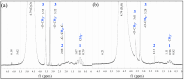
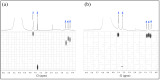
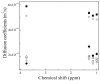
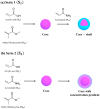
The pH- and thermo-responsive behavior of polymeric hydrogels MC-co-MA have been studied in detail using dynamic light scattering DLS, scanning electron microscopy SEM, nuclear magnetic resonance (1H NMR) and rheology to evaluate the conformational changes, swelling-shrinkage, stability, the ability to flow and the diffusion process of nanoparticles at several temperatures. Furthermore, polymeric systems functionalized with acrylic acid MC and acrylamide MA were subjected to a titration process with a calcium chloride CaCl2 solution to analyze its effect on the average particle diameter Dz, polymer structure and the intra- and intermolecular interactions in order to provide a responsive polymer network that can be used as a possible nanocarrier for drug delivery with several benefits. The results confirmed that the structural changes in the sensitive hydrogels are highly dependent on the corresponding critical solution temperature CST of the carboxylic (-COOH) and amide (-CONH2) functional groups and the influence of calcium ions Ca2+ on the formation or breaking of hydrogen bonds, as well as the decrease in electrostatic repulsions generated between the polymer chains contributing to a particle agglomeration phenomenon. The temperature leads to a re-arrangement of the polymer chains, affecting the viscoelastic properties of the hydrogels. In addition, the diffusion coefficients D of nanoparticles were evaluated, showing a closeness among with the morphology, shape, size and temperature, resulting in slower diffusions for larger particles size and, conversely, the diffusion in the medium increasing as the polymer size is reduced. Therefore, the hydrogels exhibited a remarkable response to pH and temperature variations in the environment. During this research, the functionality and behavior of the polymeric nanoparticles were observed under different analysis conditions, which revealed notable structural changes and further demonstrated the nanoparticles promising high potential for drug delivery applications. Hence, these results have sparked significant interest in various scientific, industrial and technological fields.
Keywords: (LCST) lower and (UCST) upper critical solution temperature; drug delivery systems; polyelectrolytes and particle diffusion coefficient (D); polymeric nanoparticles; stimuli-responsive hydrogels.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





 .
.

 .
.References
-
- Nidhi J., Shubam N.O., Vaibhav P., Kavet N.K. Smart materials—A state-of-the-art-review. Mater. Today Proc. 2023;82:381–389. doi: 10.1016/j.matpr.2023.03.226. - DOI
-
- Uddeshya S., Kamal G. Journey of smart material from composite to shape memory alloy (SMA), characterization and their applications-A review. Smart Mater. Med. 2023;4:227–242. doi: 10.1016/j.smaim.2022.10.002. - DOI
-
- Gong H., Yu C., Zhang L., Xie G., Guo D., Luo J. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020;202:108450. doi: 10.1016/j.compositesb.2020.108450. - DOI
-
- Prata S.A., Nascimiento R.F., Grosso C.R.F. Designing polymeric interactions toward smart particles. Curr. Opin. Food Sci. 2022;46:100867. doi: 10.1016/j.cofs.2022.100867. - DOI
-
- Hoogendboom R. Introduction to smart polymers and their applications. In: Aguilar M.R., San Róman J., editors. Smart Polymers and Their Applications. 1st ed. Elservier; Amsterdam, The Netherlands: Woodhead Publishing in Materials; Cambridge, UK: 2014. pp. 1–11. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

